CDC: First data on the side effects of the Covid-19 vaccine
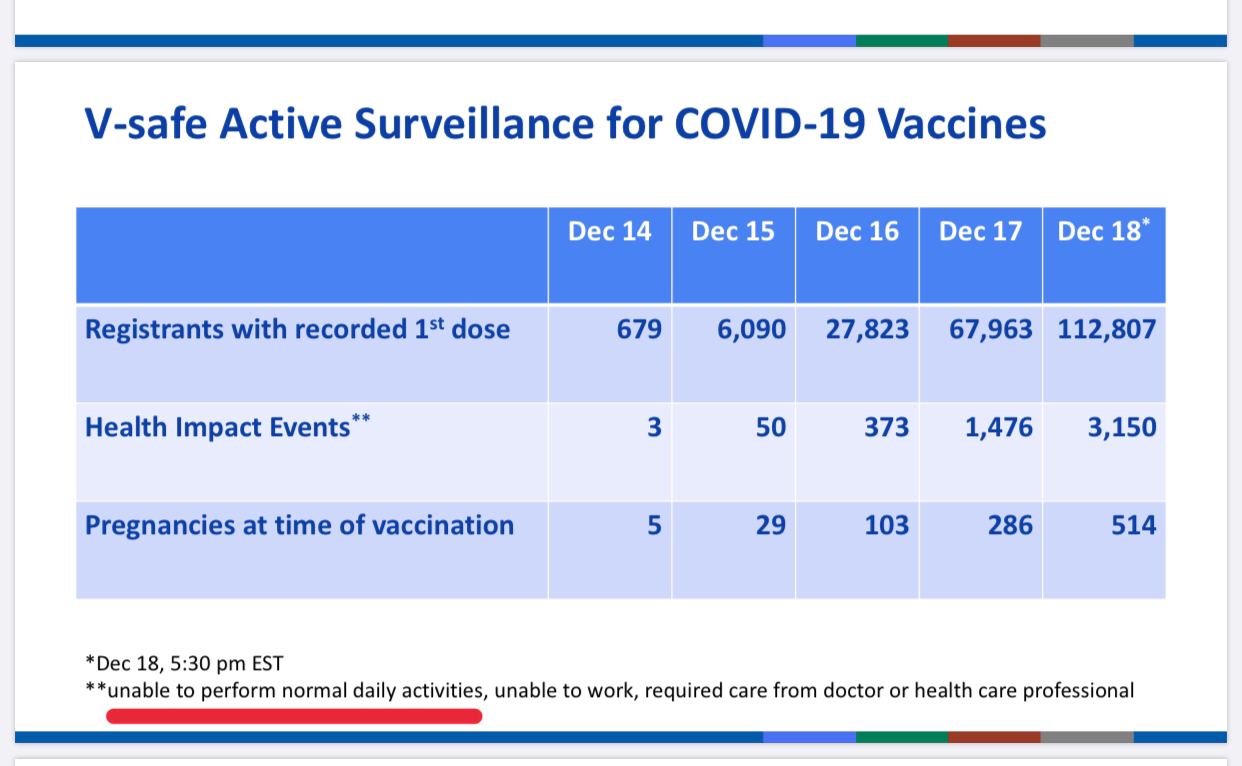
The first data published by the US CDC on 19 December 2020, which refer to the vaccination campaign in the UK, do not seem to promise anything good.
We remind you that starting from 8 December in England began the administration of the vaccine, not yet in possession of the marketing authorization, produced by the Pfizer company.
Out of 112.807 administrations as of December 18, 2020, as you can see below, 3.150 were the severe reactions that determined "inability to perform normal daily activities, inability to work, and needed medical or health care."
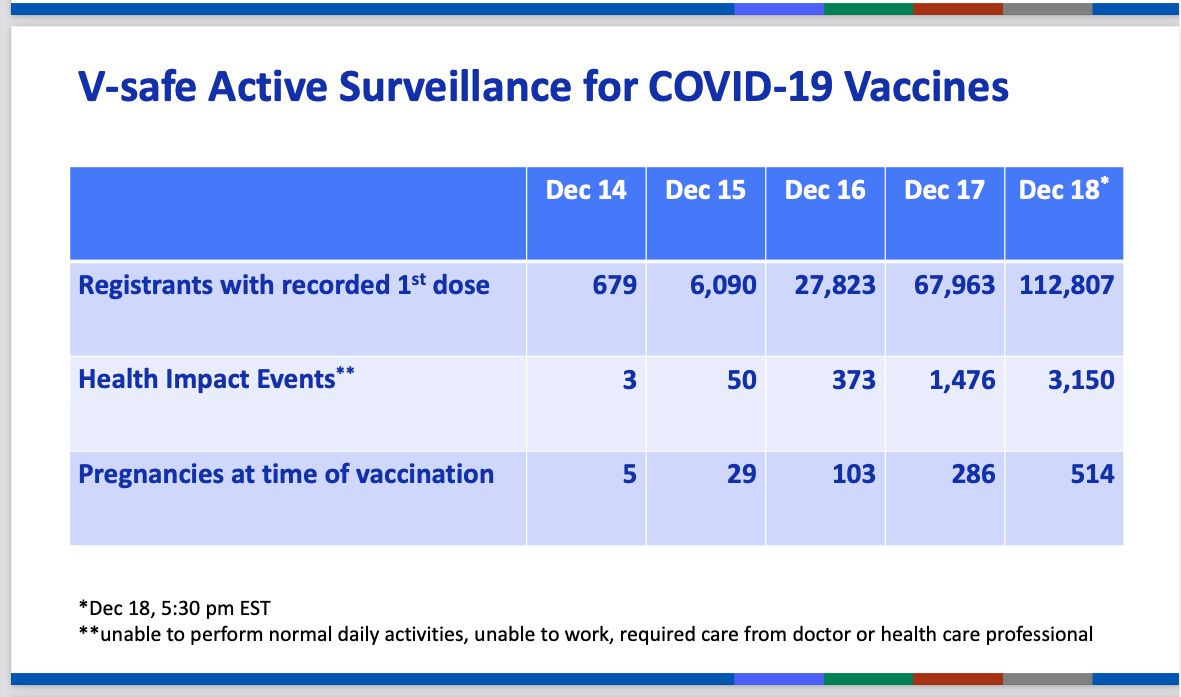
In practice, 2,8% of vaccine recipients reported disabling health problems, so much so that he is unable to work or carry out even the slightest normal activities in daily life.
The report actually focuses in particular on the 6 anaphylactic reactions reported so far, citing the actions taken by the CDC in this regard: coordination with the FDA, discussions with experts, control agencies and other subjects "in the field", the publication of a document containing considerations on the management of the risk of anaphylaxis in vaccination centers (which you find , promising) ...
But in the report another discordant note also emerges: there are 514 pregnancies in progress in vaccinated, which is not an indifferent number for a vaccine that has been contraindicated for pregnant women, and after which it is not recommended to start a pregnancy for at least three months after administration (according to the recommendations of the British JCVI vaccination committee). It must have been an accident, an oversight, or rather 514 oversights, the fact is that the vaccine is contraindicated in pregnancy and breastfeeding, yet it was evidently administered to pregnant women.
In fact, the Joint Committee on Vaccination and Immunization (JCVI extension) English published his remarks updated to December 3, before the start of the vaccine administration in the country, and reports:
Pregnancy - There are still no data on the safety of COVID-19 vaccines in pregnancy, either from human or animal studies. Given the lack of evidence, JCVI favors a precautionary approach and currently does not recommend COVID-19 vaccination in pregnancy. Women should be advised not to come forward for vaccination if they may be pregnant or are planning to become pregnant within three months of the first dose. Data is expected to inform discussions on vaccination in pregnancy. JCVI will review them as they become available.
Here, let's say that soon these data will be available, at least for 514 cases.
We recall that, however, pharmacokinetic studies are never conducted, indeed it is better to say that they are never reported, as they are not required for the authorization of vaccines that are defined as "ethical" drugs.
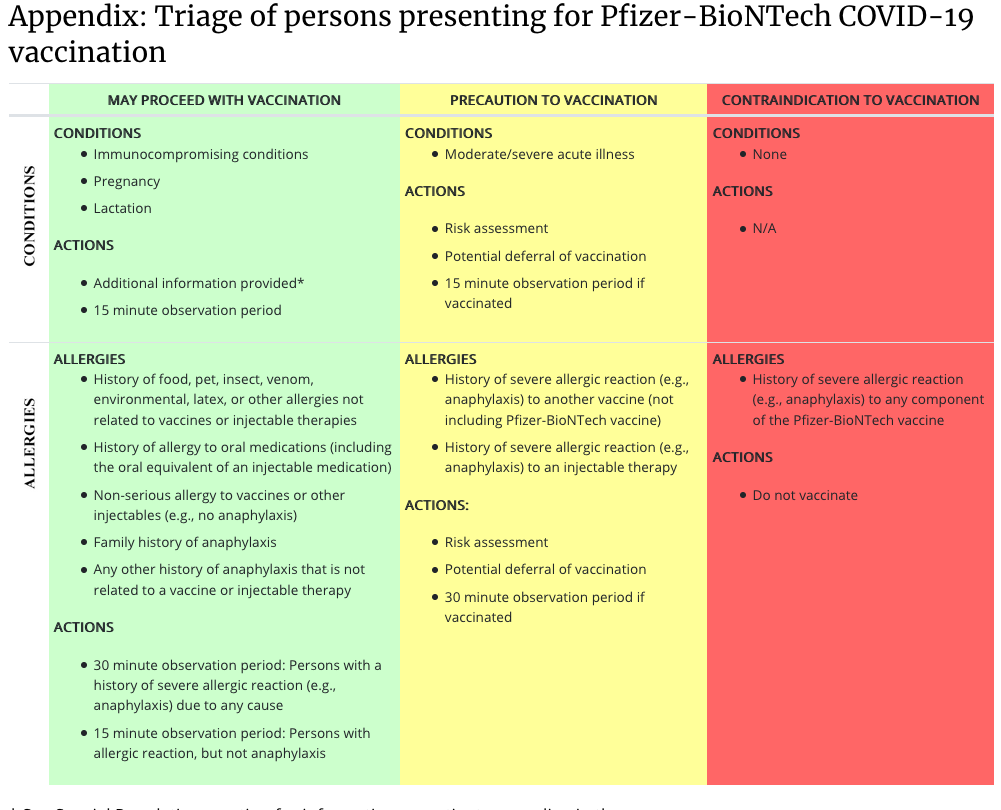
However, the CDC surprisingly included pregnant women among the subjects who should receive the vaccine (according to their will, their goodness), in its December 12 document. "Interim Clinical Considerations for Use of Pfizer-BioNTech COVID-19 Vaccine" published by ACIP:
: 
Source: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-12/slides-12-19/05-COVID-CLARK.pdf

