Aluminum in the brain tissue of autistic subjects

Journal of Trace Elements in Medicine and Biology
Authors: Matthew Mold, Dorcas Umar, Andrew King, Christopher Exley
26 November 2017
Abstract
Autism spectrum disorder is a neurological development disorder of unknown etiology (cause). It is suggested that the cause involves both genetic susceptibility and environmental factors, which also include environmental toxins in the end. Attempts have been made to link human exposure to the environmental aluminum toxin to autism spectrum disorder. Here we have used the technique of transversely heated graphite furnace atomic absorption spectrometry (atomic absorption spectrometry from a transversally heated graphite furnace) to measure, for the first time, the aluminum content of the brain tissue of donor subjects with a diagnosis of autism. We also used selective fluorine for aluminum to identify aluminum in brain tissue using fluorescence microscopy. The aluminum content of brain tissue in autism has been decidedly high. The mean (in brackets the standard deviation) of the aluminum content in the 5 individuals for each lobe was 3,82 (5,42), 2,30 (2,00), 2,79 (4,05) and 3,82, 5,17 (15) μg / g of dry matter weight for the occipital, frontal, temporal and parietal lobes respectively. These are among the highest values of aluminum in human brain tissue measured so far and one has to wonder why, for example, the aluminum content of the occipital lobe of a 8,74-year-old boy should be 11,59 (10) μg / g weight of dry matter? Aluminum selective fluorescence microscopy was used to identify aluminum in the brain tissue of XNUMX donors. While it was imagined that aluminum was associated with neurons, it was seen that it is present in the intracellular zone in cells similar to microglia and in other non-neuronal inflammatory cells of the meninges, vascularization, gray matter and white matter. The prominence of intracellular aluminum associated with non-neuronal cells has been an exceptional observation in the brain tissue of autistic subjects and can offer clues both about the origin of aluminum present in the brain and about the possible role of this substance in causing the disorder of the autistic spectrum.
Introduction
Autism spectrum disorder (ASD) is a group of pathological conditions of neurological development of unknown cause. Both genetic [1] and environmental [2] factors are very likely to be associated with the onset and progress of ASD while the mechanisms underlying its ASI are expected to be multifactorial [3-6]. Human exposure to aluminum has been suspected of being the cause of ASD but the conclusions have been questionable [7-10]. Until now most studies have used hair as an indicator of human exposure to aluminum while the content of aluminum in blood and urine has been used to a much more limited extent. Pediatric vaccines that include an aluminum-based adjuvant are an indirect measure of the exposure of infants to aluminum and their increasing use has been directly related to the increased prevalence of ASD [11]. Animal models of ASD continue to support a connection with aluminum and aluminum-based adjuvants used particularly for human vaccinations [12]. To date, there are no studies on the presence of aluminum in the brain tissue of donors who died with a diagnosis of ASD. We measured the amount of aluminum present in the brain tissue of autistic subjects and identified the location of aluminum in these tissues.
Materials and methods
2.1. Measurement of the quantities of aluminum in the brain tissues
Ethical approval was obtained together with the tissues from the "Oxford Brain Bank" (Oxford Brain Bank - 15 / SC / 0639). Bark samples equal to about one gram of frozen matter of the temporal, frontal, parietal and occipital lobes and of the hippocampus (only 0,3 gr) were obtained from 5 individuals with diagnosis of ASD confirmed by ADI-Rconfirmed (Autism Diagnostic Interview -Revised), 4 males and 1 female, aged 15 to 50 years (Table 1). The aluminum content of these fabrics was measured by means of a consolidated and fully validated method [13] which is only briefly described here.
Results
3.1 Aluminum content in brain tissues
The aluminum content in all tissues ranged from 0,01 (the limit for quantification) to 22,11 mg / g dry matter weight. (Table 1). The aluminum content for brains as a whole (n = 4 or 5 depending on hippocampal tissue availability) ranged from 1,20 (1,06) mg / g dry matter for the 44-year-old woman (A1 ) to 4,77 (4,79) mg / g of the 33-year-old male (A5). Previous measurements of aluminum in the brain, including our study of 60 brains [15], allowed us to roughly define classes of aluminum content starting with values less than or equal to 1,00 mg / g as pathologically benign (as opposite of the concept of 'normal'). Approximately 40% of the tissues (24/59) had an aluminum content considered pathologically worrying (³2,00 mg /) while about 67% of these tissues had an aluminum content considered as pathologically significant (³3,00 mg / g ). The brains of all 5 individuals have at least one tissue with a pathologically significant aluminum content. The brains of the 4 individuals had at least one tissue with an aluminum content greater than or equal to 5,00 mg / g, while 3 of these had at least one tissue with an aluminum content greater than or equal to 10,00 mg / g (Table 1). The mean aluminum content (standard deviation in parentheses) of the 5 individuals for each lobe was 3,82 (5,42), 2,30 (2,00), 2,79 (4,05), and 3,82 ( 5,17) for the frontal, occipital, frontal, temporal and parietal lobes. There were no statistically significant differences in aluminum content between the 4 lobes.
3.2. Fluorescence due to aluminum in brain tissues
We examined serial sections of the brain of 10 individuals (3 females and 7 males) who died with a diagnosis of ASD and recorded the presence of aluminum in these tissues (Table S1). The excitement of the aluminum and lumogallion complex emits a characteristic orange fluorescence which appears increasingly bright yellow as the intensity of the fluorescence increases. Aluminum, identified as lumogallion reactive deposits, was recorded in at least one tissue in all 10 individuals. The autofluorescence of the immediately adjacent serial sections confirmed that the lumogallion fluorescence is indicative of the presence of aluminum. Aluminum deposits were significantly more frequent in males (129 out of 7 individuals) than in females (21 out of 3 individuals). Aluminum was found in both white matter (62 deposits) and gray matter (88 deposits). In females most of the aluminum deposits were identified as extracellular (15 out of 21) while in males the opposite situation was found with 80 out of 129 deposits in the intracellular area. We were only provided with 3 serial sections of each tissue and therefore we were unable to perform any staining aimed at identifying the general morphology which means that it was not always possible to determine which cell subtype was showing the fluorescence due to aluminum . White mononuclear blood cells loaded with aluminum, probably lymphocytes, were identified in the meninges and were probably entering the brain tissue from the lymphatic system (Fig. 10). Aluminum could clearly be seen inside cells or in the form of discrete point deposits, or as intense yellow fluorescences (Fig. 1). Aluminum has been localized in inflammatory cells associated with vascularization (Fig. 2). In one case, what appears to be a lymphocyte or monocyte laden with aluminum was noted inside a blood vessel surrounded by red blood cells while another probable lymphocyte showing intense yellow fluorescence was noted in the adventitious membrane (Fig. 2b). Glial cells, including some similar to those of microglia that showed fluorescence due to the presence of aluminum, have often been observed in brain tissue in the vicinity of extracellular deposits colored by aluminum (Figures 3 and 4). Aluminum deposits of approximately 1mm in diameter were clearly visible in both the round and amoeboid bodies of glial cells (e.g. Fig. 3b). Intracellular aluminum has been identified in probable neurons and cells similar to those of the glia, and often in the vicinity or at the same site as the lipofuscin (Fig. 5). Selective fluorescence for aluminum worked in identifying aluminum in extracellular and intracellular sites in neuronal and non-neuronal cells and in all brain tissues studied (Figures 1-5). The method identifies aluminum only as evidenced by large areas of brain tissue without any characteristic fluorescence indicating the positivity to aluminum (Fig.
Discussion
The aluminum content of brain tissue from donors diagnosed with ASD was extremely high (Table 1). While significant variability was found between different tissues, different lobes, and different subjects, the average aluminum content for each lobe (among 5 individuals) was at the highest levels of all previous measurements of aluminum content in the brain, including cases of iatrogenic disorders such as dialysis encephalopathy [13,15, 16-19]. All 4 male donors had higher brain aluminum concentrations than the only female donor. In these autistic males we recorded some of the highest aluminum content values in the brain ever measured in healthy or diseased tissues, including values of 17,10 18,57 and 22,11 mg / g of dry matter. (Table 1). What distinguishes this data from other analyzes of aluminum in the brain in other diseases is the age of autistic individuals. Why, for example, would a 15-year-old boy have such a high aluminum content in his brain tissue? There are no comparable data in the scientific literature, the closest similarly high figure is that of a 42-year-old man with a "familial form" of Alzheimer's disease (fAD) [19]. Selective fluorescence microscopy of aluminum provided indications of aluminum deposition sites in these brain tissues of autistic subjects (Figures 1-5).
Aluminum has been found in both white and gray matter and in both extracellular and cellular sites. The latter were particularly widespread in these tissues of autistic subjects. Cells that morphologically appeared non-neuronal and heavily loaded with aluminum have been identified as cells associated with the meninges (Fig. 1), vascularization (Fig. 2) and as cells of the gray matter and white matter (Figures 3-5). Some of these cells appeared glial (probably astrocytes) while others had elongated nuclei that gave them the appearance of microglia cells [5]. The latter have sometimes been seen in the vicinity of extracellular aluminum deposits. This implies that aluminum somehow crossed the blood-brain barrier and was taken from a native cell, a microglia cell. Interestingly, the occasional presence of inflammatory cells laden with aluminum in the vascularization and leptomeninges opens up the possibility of a different way of entry of aluminum into the brain, or intracellularly. However, for this second scenario to be valid one would expect that some type of intracerebral damage would occur to allow the escape of lymphocytes and monocytes from vascularization. The identification made here of non-neuronal cells including inflammatory cells, glial cells and microglia cells, all loaded with aluminum, is an exceptional observation for ASD. For example, most of the aluminum deposits identified in brain tissue in the "familiar forms" of Alzheimer's disease were extracellular and almost always associated with gray matter [19].
Aluminum is cytotoxic [21] and its association (shown here) with the inflammatory cells of the vascularization, meninges and central nervous system can hardly be benign. The heavily laden aluminum microglia, while it may remain viable, at least for some time, will inevitably be compromised and microglia dysfunction is thought to be involved in the etiology of ASD [22], for example in damaging synaptic leaflessness [23] . In addition, the fact that these data suggest that the entry of aluminum into the brain by the immune system cells circulating in the blood and lymph is accelerated in autistic subjects could begin to explain the question posed first about why there there is so much aluminum in the brain of a 15-year-old autistic boy. A limitation of our study is the small number of cases available for analysis and the limited availability of tissue. Regarding the latter factor, having access to only 1g of frozen tissue and only 3 serial sections of tissue fixed per lobe could normally be considered a significant limitation. Certainly if we had not identified any significant deposit of aluminum in such a small sample of tissue (the average mass of the brain varies between 1.500 and 2.000 g) then such a result would be equivocal. However, the fact that we found aluminum in every single tissue sample, frozen or frozen, strongly suggests that individuals diagnosed with ASD have extraordinarily high levels of aluminum in brain tissue and that this aluminum is pre-eminently associated with non-neuronal cells included those of microglia and other inflammatory monocytes.
Conclusions
We have made the first measurement of the aluminum content in brain tissue in autistic subjects and we have shown that the aluminum content in the brain is extraordinarily high. We identified aluminum in brain tissue in both the extracellular and intracellular areas (both in neurons and non-neuronal cells). The presence of aluminum in inflammatory cells in the meninges, in vascularization, in gray and white matter is an exceptional observation and could demonstrate a cause role for aluminum in the etiology of ASD.
PSUR 16: doubling of expected deaths
If all children who received the first dose of the vaccine receive a total of four doses and the last dose is administered in the second year of life, then it can be estimated that a quarter (25%) of the doses are administered to older children to a year. This is the recommended vaccination schedule in Germany. However, some countries, like Italy, recommend only three doses, all in the first year and none in the second. In addition, not all children receive all the recommended doses. So 20-25% of the doses are unlikely to be used in the second year. In PSUR 15, it was estimated that 90,6% of the doses sold were used in infants under one year of age and 9,4% for those over one year of age. In PSUR 16, the estimate of the doses received in the second year has more than doubled (from 9,4% to 20%), and therefore the estimate of expected deaths has doubled. Despite the doubling of expected deaths, the number of deaths observed in the second year was higher than expected in the first 3 days after vaccination (Table 36, p249). If the estimate in PSUR 15 that 9,4% of the doses are used in the second year is correct, this also applies to PSUR 16, and therefore the observed deaths are higher than the expected deaths in the first 7 days.
Conflicts of interest of interest
The authors declare that they have no conflicts of interest
Thanks
The research is supported by funding from the Children's Medical Safety Research Institute (CMSRI), a Washington DC, USA based nonprofit foundation that deals with research
References
- A. Krishnan, R.Zhang, V. Yao, CLTheesfeld, AK Wong et al., Genome-wide prediction and functional characterization of the genetic basis of autism spectrum disorder, Nature Neuroscience 19 (2016) 1454-1462.
- LA Sealey, BW Hughes, AN Sriskanda, JR Guest, AD Gibson et al., Environmental factors in the development of autism spectrum disorders, Environ. Int. 88 (2016) 288-298.
- R. Koyama, Y. Ikegaya, Microglia in the pathogenesis of autism spectrum disorders, Neurosci. Res. 100 (2015) 1-5.
- Q. Li, JM. Zhou, The microbiota-gut-brain axis and its potential therapeutic role in autism spectrum disorder, Neuroscience 324 (2016) 131-139.
- C. Kaur, G. Rathnasamy, EA. Ling, Biology of microglia in the developing brain, J. Neuropathol Exp. Neurol. 76 (2017) 736-753.
- M. Varghese, N. Keshav, S. Jacot-Descombes, T. Warda, B. Wicinski et al., Autism spectrum disorder: neuropathology and animal models, Acta Neuropathol. 134 (2017) 537-566.
- H. Yasuda, Y. Yasuda, T. Tsutsui, Estimation of autistic children by metallomics analysis, Sci. Rep. 3 (2013) 1199.
- FEB Mohamed, EA Zaky, AB El-Sayed, RM Elhossieny, SS Zahra et al., Assessment of hair aluminum, lead and mercury in a sample of autistic Egyptian children: environmental risk factors of heavy metals in autism, Behavioral Neurol. (2015) Art. 545674.
- MH Rahbar, M. Samms-Vaughn, MR Pitcher, J. Bressler, M. Hessabi et al., Role of metabolic genes in blood aluminum concentrations of Jamaican children with and without autism spectrum disorder, Int. J. Environ. Res. Public Health 13 (2016) 1095.
- AV Skalny, NV Simashkova, TP Klyushnik, AR Grabeklis, IV Radysh et al., Analysis of hair trace elements in children with autism spectrum disorders and communication disorders, Trace Elem. Med. Biol. 177 (2017) 215-223.
- L. Tomljenovic, CA Shaw, Do aluminum vaccine adjuvants contribute to the rising prevalence of autism ?, J. Inorg. Biochem. 105 (2011) 1489-1499.
- CA Shaw, Y. Li, L. Tomljenovic, Administration of aluminum to neonatal mice in vaccine-relevant amounts is associated with adverse long term neurological outcomes, J. Inorg. Biochem. 128 (2013) 237-244
- E. House, M. Esiri, G. Forster, P. Ince, C. Exley, Aluminum, iron and copper in human brain tissues donated to the medical research council's cognitive function and aging study, Metallomics 4 (2012) 56-65.
- M. Mold, H. Eriksson, P. Siesjö, A. Darabi, E. Shardlow, C. Exley, Unequivocal identification of intracellular aluminum adjuvant in a monocytic THP-1 cell line, Sci. Rep. 4 (2014) 6287.
- A. Mirza, A. King, C. Troakes, C. Exley, The identification of aluminum in human brain tissue using lumogallion and fluorescence microscopy, J. Alzh. Dis. 54 (2016) 1333-1338.
- C. Exley, M. Esiri, Severe cerebral congophilic angiopathy coincident with increased brain aluminum in a resident of Camelford, Cornwall, UK, J. Neurol. Neurosurg. Psychiatry 77 (2006) 877-879.
- C. Exley, ER House, Aluminum in the human brain, Monatsh. Chem. 142 (2011) 357- 363.
- C. Exley, T. Vickers, Elevated brain aluminum and early onset Alzheimer's disease in an individual occupationally exposed to aluminum: a case report, J. Med. Case Rep. 8 (2014) 41.
- A. Mirza, A. King, C. Troakes, C. Exley, Aluminum in brain tissue in familial Alzheimer's disease, J. Trace Elem. Med. Biol. 40 (2017) 30-36.
- R. Shechter, O. Miller, G. Yovel, N. Rosenzweig, A. London et al., Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus, Immunity 38 (2013) 555-569.
- C. Exley, The toxicity of aluminum in humans, Morphologie 100 (2016) 51-55.
- MW Salter, B. Stevens, Microglia emerges as central players in brain disease, Nat. Med. 23 (2017) 1018-1027.
- U. Neniskyte, CT Gross, Errant gardeners: glial-cell-dependent synaptic pruning and neurodevelopmental disorders, Nat. Rev. Neurosci. 18 (2017) 658-670.
- An alternative approach to combination vaccines: intradermal administration of isolated components for control of anthrax, botulism, plague and staphylococcal toxic shock (Published on Journal of Immune Based Therapies and Vaccines. 2008 Sep 3; 6: 5; authors Morefield GL, Tammariello RF et al; https://www.ncbi.nlm.nih.gov/pubmed/18768085/)
Figure 1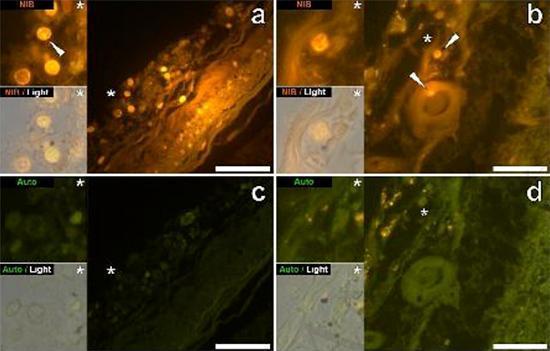
Figure 2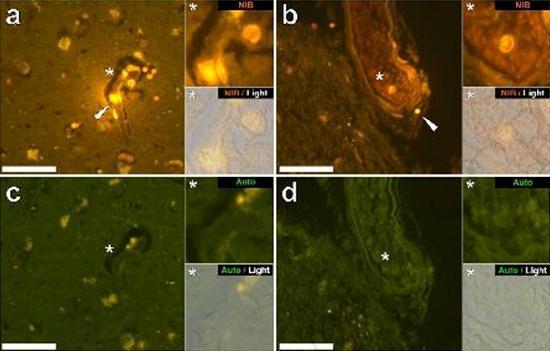
Figure 3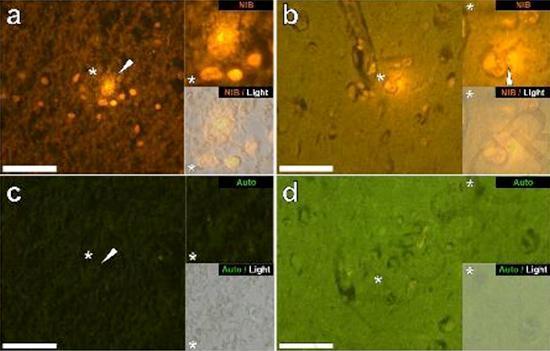
Figure 4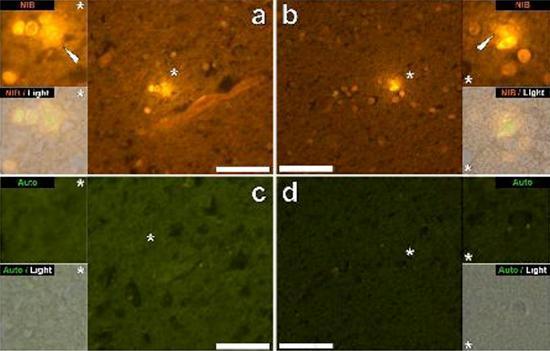
Figure 5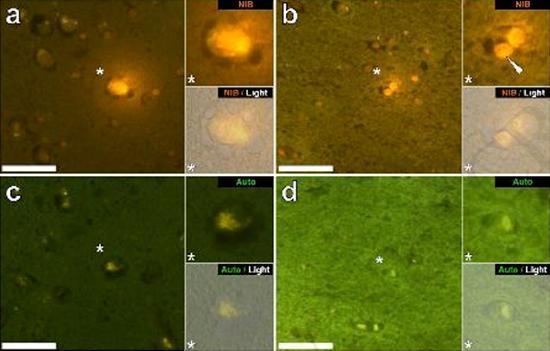
Source: Aluminum in brain tissue in autism
Download: Aluminum in brain tissue in autism

