Infanrix hexa and sudden death: a review of the update of the periodic safety reports submitted to the European Medicines Agency

The vaccine in question, "Infanrix hexa", which combines diphtheria, tetanus, pertussis, hepatitis B, polio and type B flu vaccines, is produced by GlaxoSmithKline (GSK) and was introduced in Europe in October 2000.
Puliyel and Sathyamala discovered the concealment by analyzing the data in the periodic safety update reports (PSUR) related to the vaccine that its manufacturer GSK must regularly provide to the European Medicines Agency (EMA).
These confidential safety reports on this vaccine were received by Puliyel from an Italian researcher who obtained them from the EMA under the Freedom of Information Act - the Italian version of the right to information in India.
According to the analysis, doctors found that the latest "Infanrix hexa" vaccine safety report submitted by GSK (2015) has canceled the deaths previously reported by the manufacturer in his 16th report (2012). They note, however, that it is not clear in the report how these deaths were canceled.
Authors Puliyel and Sathyamala note that ten years after the publication of an article from the Center for Disease Control (CDC) examining the relationship between MMR and autism, one of the authors William Thompson admitted that he and his co-authors had omitted information. statistically significant - i.e. that African American males who had been given the MMR vaccine before the age of 36 months were at increased risk of autism. After Thompson and his colleagues found evidence of this increased risk, they deleted data from children without Georgian birth certificates (and therefore disqualified a disproportionate number of black children) and presented their data saying there was no increased risk of autism. It is unclear whether the authors of PSUR 19 performed a similar retroactive disqualification of children documented to have died in PSUR 16.
"If these deaths weren't cleared, the deaths after vaccination would have been significantly higher than expected by chance. The manufacturer would have had to admit to the EMA that their vaccine was the cause of those excess deaths."
Puliyel and Sathyamala claim that the producer "must explain the seemingly flawed figures that he submitted to regulatory authorities.
So far the manufacturer has claimed that the deaths reported after the vaccine are "coincident" and that they would have taken place in these children even if they had not received vaccinations.
However, in their review in the journal, Puliyel and Sathyamala point out that their analysis showed that 83% of the reported deaths occurred immediately after vaccination in the first 10 days and only 17% happened in the following ten days.
"If it were coincident deaths, then they would not have all clustered immediately after vaccination, but would have been evenly distributed over the 20-day period."
Puliyel and Sathyamala write that any argument that claims that sudden deaths after vaccination are offset by lives saved by the vaccine is not acceptable, in the same way that it would be considered illegal to kill a person to use his organs to save five other people. .
"Hiding deaths after vaccination can prevent or delay the assessment of the vaccine's safety profile and this can lead to unnecessary and ethically justifiable deaths.
The authors point out that Hexavac - a similar vaccine manufactured by Sanofi Pasteur and also introduced to the market in 2000 was withdrawn from the European market in 2005. It was found that the death of children increased within two days of vaccination.
In the Indian context, the authors point out that the Drug Controller General of India (DCGI) should reconsider the current automatic approval policy for all drugs authorized in the United States and Europe. "This reliance based on due diligence by the EMA can be wrong and needs to be reviewed."
"Pentavac", produced by the Serum Institute of India and marketed in India, is similar to the now discontinued Hexavac and Infanrix Hexa reported here, except that the whole cell pertussis vaccine is replaced by an acellular vaccine and has a sixth component, the injectable polio vaccine. "
In light of their feedback, Puliyel and Sathyamala suggest that "it is imperative that DCGI be aware of the PSUR reports provided to the EMA and the concerns raised through this comment."
Clinic
Indian Journal of Medical Ethics Online First Published September 5, 2017
Infanrix hexa and sudden death: a review of the update of the periodic safety reports submitted to the European Medicines Agency
JACOB PULIYEL, C SATHYAMALA
Authors: Jacob Puliyel (corresponding author -
To cite: Puliyel J, Sathyamala C. Infanrix hexa and sudden death: a review of the periodic safety update reports submitted to the European Medicines Agency. Indian J Med Ethics. Published online on September 5, 2017. Corrected on September 9, 2017 *. DOI: 10.20529 / IJME.2017.079
Manuscript Editor: Mala Ramanathan © Indian Journal of Medical Ethics 2017
Abstract
There have been several spontaneous reports of unexpected sudden death immediately after the administration of Infanrix hexa (combined diphtheria, tetanus, acellular pertussis vaccine, inactivated poliomyelitis, hepatitis B and Haemophilus influenzae type B). The manufacturer, GlaxoSmithKline (GSK), submits periodic confidential safety update reports (PSURs) on Infanrix Hexa to the European Medicines Agency (EMA). The last is PSUR * 19. Each PSUR contains an analysis of observed / expected sudden deaths, which shows that the number of deaths observed immediately after immunization is less than expected by chance.
This comment focuses on that aspect of the PSUR that affects political decisions. We have analyzed the data provided in the PSUR. It is evident that the deaths recognized in PSUR 16 have been eliminated by PSUR 19. The number of deaths observed immediately after vaccination in children over one year of age was significantly higher than expected by chance once the cleared deaths were restored and included in the analysis.
The manufacturer must explain the figures that have been submitted to the regulatory authorities. The procedures undertaken by the EMA to evaluate manufacturer's alerts in the PSUR need to be reviewed. The Medicines Controller General of India almost automatically accepts EMA approved drugs and vaccines. There is a need to review confidence in diligence by the EMA.
Introduction
On 23 October 2000, the marketing of two hexavalent vaccines, Infanrix hexa® (GlaxoSmithKline plc-GSK) and Hexavac® (Sanofi Pasteur MSD, SNC), which combine diphtheria, tetanus, acellular pertussis, hepatitis B, la inactivated poliomyelitis and influenza Haemophilus type B has been authorized in the European Union. After authorization, there were several spontaneous reports of unexpected sudden death immediately following the administration of these hexavalent vaccines. In 2005 von Kries and colleagues (1) performed a detailed analysis in which they compared the deaths observed immediately after vaccination with the expected casualties. They found that the standardized mortality ratio (SMR) within two days of Hexavac vaccination had significantly increased in children vaccinated in the second year of life.
This was not the case with Infanrix Hexa. At the request of the marketing authorization holder, Hexavac was withdrawn in 2005 and Infanrix Hexa continued to be marketed in Europe (2). According to European law, the European Medicines Agency (EMA) is responsible for protecting public health through the evaluation of medicines approved by it as regulatory authorities. Manufacturers are responsible for the efficacy, quality and safety of their drugs (3).
The Italian court of judge Nicola Di Leo has made available to the public the periodic confidential security update reports (PSUR) 15 and 16a from 2009 to 2011 of GlaxoSmithKline (4). The PSUR 19 (which incorporates the PSUR 17, 18 and 19, dated 15 January 2015) was obtained by an Italian researcher from the EMA pursuant to article 3 of the EMA rules (EMA 110196/2006 of 30 November 2010) (5 ). The Italian doctor sent this PSUR to the first author (JP), asking him to write a report to be presented to the European Parliament. This commentary is based on all these PSURs. In the context of the safety aspect previously highlighted by von Kries (1), this comment examines sudden death after the use of the Infanrix Hexa vaccine. Other aspects covered in the PSUR are not examined.
PSUR 15 - grouping of dead after vaccination
Most deaths that occur in the post-neonatal period are due to infections, birth defects, malignancies or accidents. Children rarely die without an obvious cause and so deaths are classified as (i) sudden death syndrome (SIDS), defined in the PSUR as death that occurs in the first year of life and remains unexplained after the autopsy, or (ii ) unexpected sudden death (SOUTH), defined as the death that occurs within the first two years of life, and which remains unexplained after clinical and final history of events, but without an autopsy. Together, these two are considered to be sudden death (SD) in PSUR 15. A certain number of vaccines are given to children under 2 years old on a given day, and the number of children vaccinated worldwide is very large. It is possible that some vaccinated children could accidentally die of SIDS / SOUTH incidentally, such events would have occurred even if these children had not been vaccinated on that day. To ascertain whether such a death was caused by vaccination or was an incidental event, an observed / expected SD analysis is performed. The analysis assesses whether the number of deaths observed after vaccination exceeds what can be predicted by chance.
Sudden deaths: observed towards expectations
PSUR 15 explains how this analysis was performed (4: p 782): "The Society evaluated whether the number of sudden deaths reported in this age group that exceeded number one could occur randomly. Since the age distribution in which subjects were vaccinated is unknown, the company assumed that the proportion of adverse events by age is representative of the actual age distribution at vaccination. It can therefore be estimated that 90,6% of all Infanrix recipients Hexa were in their first year of life, and 9,4% were in the second year of life. Hence the number of doses (since launch) was estimated to be 54.927.729 and 5,698,904, respectively. As Germany is the main country where Infanrix Hexa doses are distributed (almost 30% in Germany alone), the incidence of sudden death observed in Germany was assumed to be representative of the entire recipient population of Infanrix Hexa (Federal OfficeGerman Statistician, Statistisches Bundesamt; incidence rate in the first year of life: 0,454 / 1000 live births; second year: 0.062 / 1000 live births, 2008 data).
"The PSUR documents the reported death within 20 days of vaccination. The observed death toll was lower than expected (table 1).
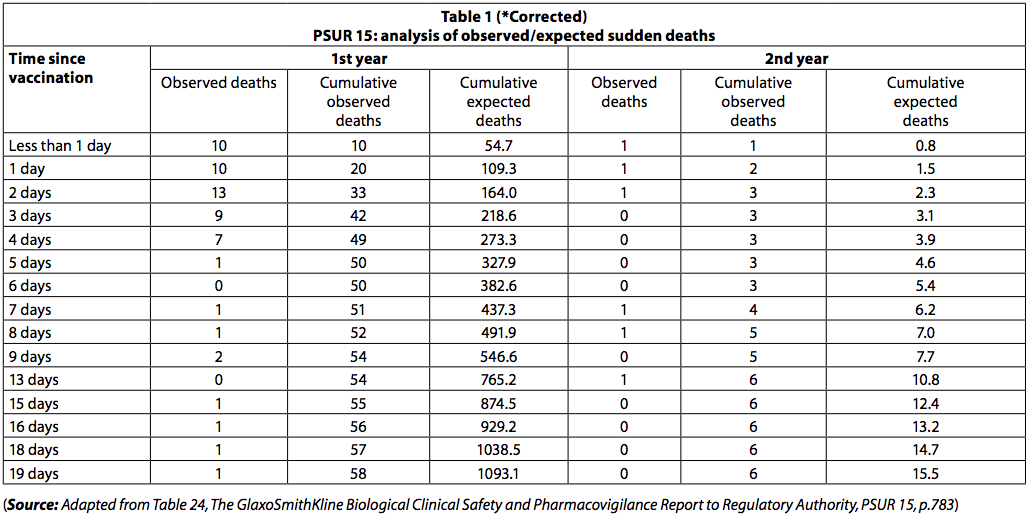
However, among newborns, there was a clustering of deaths immediately after vaccination, with 42 deaths occurring in the first three days after vaccination and only 8 in the next 3 days. Among those less than one year old, 54 deaths (93%) occurred in the first ten days and 4 (7%) in the following 10 days. If the deaths had been "random SIDS deaths", this disparity in the number of deaths over the two time periods would not have been observed.
Deaths from SIDS would have been evenly distributed over the 20 day period.
The fact that the death rate drops rapidly with the passage of time following immunization suggests that the deaths may be related to vaccination.
Similarly, among children over the age of one year, 5 deaths (83,3%) occurred in the first 10 days and one death (17%) occurred in the following 10 days. The grouping of deaths reported in PSUR 15 was also noted in PSUR 16, and this has been commented on earlier (6).
GlaxoSmithKline's answer
In response to this criticism (7), GlaxoSmithKline (GSK) Chief Executive Officer (CEO) Sir Andrew Witty, through Dr. Norman Begg, the company's Chief Medical Officer, suggested in a letter that there is much more likely to think of a potential causal association and then to report an adverse event to GSK if it occurs shortly after vaccination rather than weeks later. He further wrote: "In light of the above, we remain confident in the conclusions previously reached by GSK and shared with regulatory agencies and health authorities around the world, that the currently available data does not suggest an increased risk of sudden infant death after vaccination with Infanrix hexa. Should available data and information change and suggest that there is an increased risk, we remain committed to promptly notify authorities and take the necessary action to communicate such data and information to healthcare professionals. "
This response contains a tacit admission that there was no active vigilance during the post-vaccination period and only deaths spontaneously reported to GSK were included under the heading "observed deaths". This could cause an underestimation of deaths after vaccination. It should be noted that the number of distributed vaccine doses is used for "expected deaths". The report acknowledges that all doses of the distributed vaccine have not necessarily been used. In this way, the numbers of "expected deaths" were inflated.
However, given the CEO's explanation and assurance that GSK has committed to promptly notifying the authorities and healthcare professionals of any increased risk with Infanrix Hexa, the issue of grouping deaths has not been further investigated.
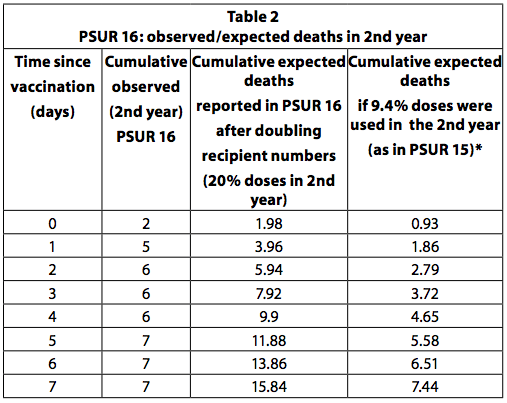
PSUR 16: doubling of expected deaths
If all children who received the first dose of the vaccine receive a total of four doses and the last dose is administered in the second year of life, then it can be estimated that a quarter (25%) of the doses are administered to older children to a year. This is the recommended vaccination schedule in Germany. However, some countries, like Italy, recommend only three doses, all in the first year and none in the second. In addition, not all children receive all the recommended doses. So 20-25% of the doses are unlikely to be used in the second year. In PSUR 15, it was estimated that 90,6% of the doses sold were used in infants under one year of age and 9,4% for those over one year of age. In PSUR 16, the estimate of the doses received in the second year has more than doubled (from 9,4% to 20%), and therefore the estimate of expected deaths has doubled. Despite the doubling of expected deaths, the number of deaths observed in the second year was higher than expected in the first 3 days after vaccination (Table 36, p249). If the estimate in PSUR 15 that 9,4% of the doses are used in the second year is correct, this also applies to PSUR 16, and therefore the observed deaths are higher than the expected deaths in the first 7 days.
PSUR 19: predicted deaths weighted by country and annual proportion of doses
In PSUR 19, a calendar-weighted average of the incidence rates of sudden deaths from Germany, France and the Netherlands was calculated to arrive at the expected incidence of sudden deaths.
In very simple terms, this means that if 60% of the doses were distributed in Germany in a given year, the SD (Sudden Death) rate in Germany was given a weight of 60% when calculating the SD rate. overall for that year; if 30% was distributed in France, the SD rate in France contributed 30%, and the weight of 10% was given to the Dutch SD rate. Finally, the global SD rate was calculated for all the years together.
The overall SD rate was calculated as 0.0102 / 1000 live births for the second year. This figure is one sixth of the expected frequency used in PSUR 15 and 16 (which calculated sudden deaths at 0,062 / 1000 live births, using German data).
The Poisson confidence interval (CI) of 95% of deaths observed in the second year is reported in table 8 ap 447 of the PSUR 19. It is reported that for the second year of life, the number of deaths observed was higher, also if not significantly, than that of expected deaths within a risk period of 1-4 days after vaccination.
Missing deaths in PSUR 19
From PSUR 16 to PSUR 19, total doses of the vaccine rose from 69 to 112 million. According to the PSUR 19, it was assumed that 20,2% of the distributed doses were administered to children in the second year of life (PSUR 19, pp 436-448). Cases of death in which the vaccination age was not known, the date of death was not recorded, or the time of death exceeded 19 days from vaccination, were excluded.
The PSUR 19 (deaths until 22 October 2014) does not report the sudden deaths mentioned in PSUR 16 (death cases that occurred until 22 October 2011). It should be noted that in PSUR 16 the age of the child who died after vaccination and the time of death (within 14 days of vaccination) were both recorded.
Cumulative deaths reported are lower in PSUR 19 than in PSUR 16. As for children over one year, PSUR 19 records the occurrence of only 5 deaths in the first 19 days after vaccination, while PSUR 16 reports 8 The numbers are not consistent with each other. We wonder why this is so.
Ten years after the publication of the CDC (Center for Disease Control) article examining the relationship between the measles, mumps and rubella (MMR) vaccine and autism (8), one of the authors, William Thompson, admitted that he and his co-authors failed to show statistically significant information that African-American males who received MMR before the age of 36 months had an increased risk of autism (9). The authors deleted the data of the children who did not have birth certificates in Georgia (10), in order to exclude a disproportionate number of black children, and presented their data in order to show that there had not been an increase in the risk.
It is unclear whether the authors of PSUR 19 have similarly ruled out dead and documented children in PSUR 16.
Table 3 presents the observed and expected deaths reported in PSUR 19 and the deaths observed after the restoration of the deaths reported in PSUR 16.
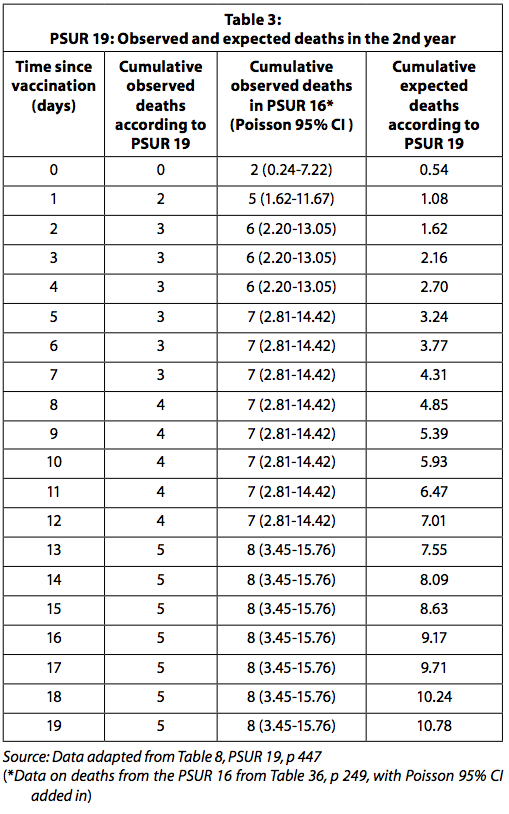
When the observed deaths data in PSUR 16 are used, the number of observed deaths is significantly higher than expected for the first four days after vaccination. It must be kept in mind, as explained above, that since the number of observed deaths is collected passively, it is likely to be underestimated. Expected deaths, on the other hand, are likely overestimated as they are calculated with the assumption that all distributed doses have been used without any loss and no vaccine has been discarded because it has expired. GSK was expected to report the statistically significant increased risk of death in the four days after vaccination to the regulatory authority and physicians.
The doses used in the second year
PSUR 19 assumes that 20,2% of the doses have been used in the second year. It states that the distribution of the age at which the subjects are vaccinated is unknown, and the company has speculated that the proportion of adverse events (including death) by age is representative of the actual age distribution at vaccination. Thus, since 20,2% of the adverse events occurred in children over one year of age, the company assumed that 20,2% of the doses have been used for this age group.
It is easy to estimate the number of doses used in the second year based on the observed adverse events (including death), then use this dose estimate to calculate the number of expected deaths, and finally compare it to the number of observed deaths - given that the estimate of expected deaths is calculated primarily from the observed negative events (including death).
Assuming that all deaths after vaccination are coincident and non-causal SIDS / SUD related to the vaccine, and given that (according to PSUR 19) the natural frequency of sudden death in the first year is 44 times higher than in the second year (0.441 / 1000 in the first year and 0.0102 / 1000 in the second year), 44 times more children should be vaccinated in the second year to reach the same number of deaths as in the first year.
In a cohort of 100 deaths, if 20% of sudden deaths occur in the second year and 80% in the first year, 880 children must be vaccinated in the second year for every 20 vaccinated in the first year. In this case, it would be assumed that 44% of all doses of Infanrix hexa would have been used in the second year and only 80% in the first year (instead of the other way around). This reflects the absurdity of calculating dose distribution by age, based on the age distribution of adverse events, as done in the GSK document.
The only way to evaluate the number of doses used in the second year is to look at vaccination schedules in different countries - looking at which countries recommend the fourth dose in the second year and which ones do not recommend the doses in the second year. A weight can be assigned for the number of doses distributed in these countries. The dropout rate (children who leave the vaccination program after receiving the first vaccine doses) must also be considered in the final calculation of the percentage of doses used in the second year. It would seem that a reasonable estimate of the doses used in the second year is 9,4% of the total doses and this is the figure used in the PSUR 15.
The ethical dilemma - the trolley problem
This comment does not attempt to examine whether these excessive deaths after vaccination (presumably caused by the vaccine) can be offset by the lives saved by the disease with the prevention obtained with the vaccine. In his classic mental experiment, called the trolley dilemma, Philippa Foot asks if it is ethical to redirect a trolley escaped from a railway track that would have killed five people on another track where only one would have died (11) . In a variant of the cart dilemma, the single person on the alternative track is the child of the person who can switch the tracks.[2] Judith Thomson assumes that five lives can be saved by organ transplants from a healthy donor, and asks if it would be ethical to intentionally kill one person to save the other five (12). Ethics argue that the end cannot justify the means. If you hide death after vaccination, you can prevent / delay the assessment of the safety profile of the vaccine and this has the potential to cause more unnecessary deaths, difficult to justify ethically.
Relevance for India
The regulatory authority of the government of India is the Drug Controller General of India (DCGI). According to DCGI rules, drugs approved in one or more countries, such as the United States, the United Kingdom, Canada, Japan, Australia and European Union countries, are also approved in India (13). Only additional studies are needed to assess the impact of ethnic factors on the efficacy, safety, dosage and dosage regimens of drugs (14).
Recently, studies examining the immunogenicity and safety of the hexavalent combination vaccine in small trials have been published in India (15,16). Additionally, Indian Pediatrics published an editorial titled "Hexavalent Vaccinations: The Future of Routine Immunization?" (17), who suggested that this combination vaccine would be promoted in India. It is vital that the regulatory authority in India is aware of the concerns raised in this commentary on PSUR reports, particularly as surveillance systems in India are weak.
Summary and conclusion
Von Kries (1) reported a statistically significant increase in SMR in children in their second year of life, within two days of vaccination with Hexavac® (one of two authorized hexavalent vaccines, now withdrawn).
In the periodic safety update reports, GSK, the production company of Infanrix hexa, assesses whether the number of sudden deaths reported after vaccination with their drug has exceeded the number that could be expected by chance. The grouping of deaths immediately after immunization suggests that the death may have been caused by the vaccine.
Furthermore, our analysis shows that the deaths recognized in PSUR 16 have been eliminated from PSUR 19. The observed deaths are reported spontaneously to GSK and are likely to be underestimated. By adding the deaths cleared from the PSUR 16, there is a statistically significant increase in the risk of death in the first four days after vaccination, compared to expected deaths. Manufacturers will need to explain why these deaths were not included in PSUR 19. The increased risk of death was not disclosed to the regulatory authority or healthcare professional who administers this vaccine.
Given the above, it is difficult to understand how EMA may have accepted PSUR 19 on a nominal basis. It can be argued that due diligence has not been exercised, so that many children have been exposed unnecessarily to the risk of death.
DCGI must be made aware of the limitations in the Infanrix Hexa PSUR.

