Ema, gel for skin treatment: cancer risk for patients using ingenol mebutate
The European Medicines Agency (Ema) aim the headlights on a gel for the treatment of a disease of skin suspected of increasing cases of cancer.
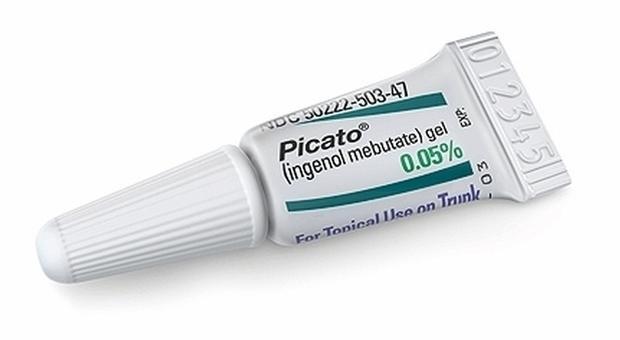
The agency has announced the revision of the data on the tumor of the skin in patients using ingenol mebutate (Picato), a gel for the treatment of actinic keratosis, skin lesion caused by excessive exposure to the sun. This is learned from the Portal of the Italian Agency of Drug (AIFA). The review, it says, "was initiated on the basis of data from several studies showing a higher number of cases of skin cancer, including cases of squamous cell carcinoma, in patients using this gel."
In order to determine whether or not Picato increases the risk of skin cancer, the Ema Safety Committee (Prac) will carry out a thorough review of all available data, including on the basis of ongoing studies. Upon completion, it will recommend whether the marketing authorization for the medicinal product in the EU should be changed. In the meantime, healthcare professionals are advised to "use Picato with caution in patients who have previously had skin cancer. In addition, patients should continue to pay attention to any skin lesions and notify the doctor immediately if they notice anything unusual. "

