MMR vaccine (Measles-Mumps-Rubella)

1. What are the side effects of the MMR vaccine?
Common side effects of the MMR vaccine include fever, mild rash, and swollen glands in the cheek or neck.1 A more serious side effect is seizure, which occurs in about 1 in 640 children vaccinated with MMR.2 - about five times more often than the seizure due to measles infection.3
The World Health Organization (WHO) states that severe allergic reactions to the vaccine occur in about 1 in 100.000 doses.4 However, other serious side effects include deafness, long-term seizures, coma, lowered consciousness, permanent brain damage, and death.1 While the CDC says these side effects are rare, the exact numbers are unknown.1 Additionally the manufacturer's package insert states that "MMR II has not been evaluated for carcinogenic or mutagenic potential, or for potential to impair fertility".5
2. How are the risks of vaccine side effects measured?
Methods for measuring vaccine risks include surveillance systems, clinical trials, and epidemiological studies.
3. How accurate is the surveillance for adverse events of the MMR vaccine?
The US government tracks reported cases of vaccine side effects through the Vaccine Adverse Event Reporting System (VAERS). Approximately 40 deaths and permanent injuries caused by MMR vaccine are reported to VAERS each year.6 However, VAERS is a passive reporting system - authorities do not actively seek cases and do not actively remind doctors and the public to report them. These limitations can lead to significant under-reporting.7 The CDC states that "VAERS only receives reports for a small fraction of actual adverse events".8 In fact, only 1% of serious side effects from medical products are reported to passive surveillance systems9 and only 1,6% of MMR vaccine-related seizures are reported to VAERS.10 Additionally, VAERS reports are not proof that a side effect has occurred, as the system is not designed to thoroughly investigate all cases.11 Consequently, VAERS does not provide an accurate count of MMR vaccine side effects.
Potential risk of permanent injury from MMR vaccine vs. risk of death from measles
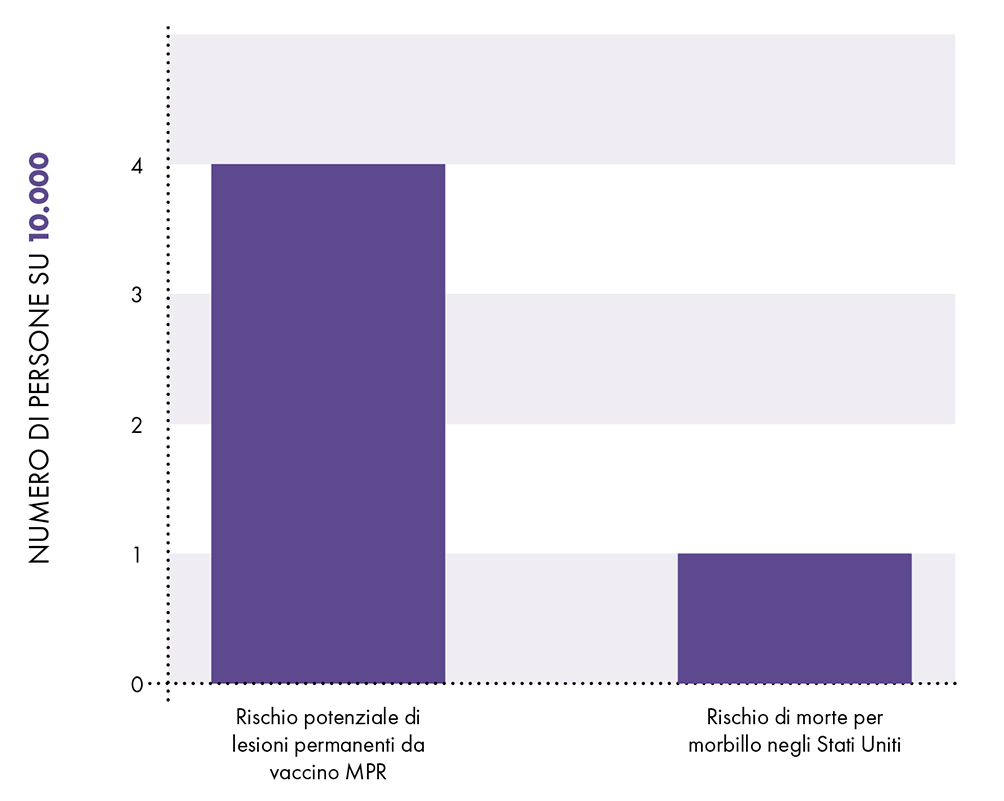
Figure 1: A 2002 Danish study did not rule out the possibility that the MMR vaccine can cause an adverse event with permanent lesions four times more often than measles can be fatal.
4. How accurate are clinical trials of the MMR vaccine?
The CDC states, "Pre-licensed studies are relatively small - usually limited to a few thousand subjects - and usually last no more than a few years. Since measles is fatal in about 1 in 10.000 cases and causes permanent injury in about 1 in 80.000. XNUMX case in XNUMX,3 a few thousand subjects in clinical trials are not sufficient to demonstrate that MMR vaccine causes fewer permanent deaths and lesions than measles (Fig. 1). Furthermore, the lack of adequate clinical trials on the MMR vaccine meant that the manufacturer's package insert data were based on passive surveillance for rates of neurological adverse reactions, permanent disability and MMR-related death.5
A limitation of clinical trials
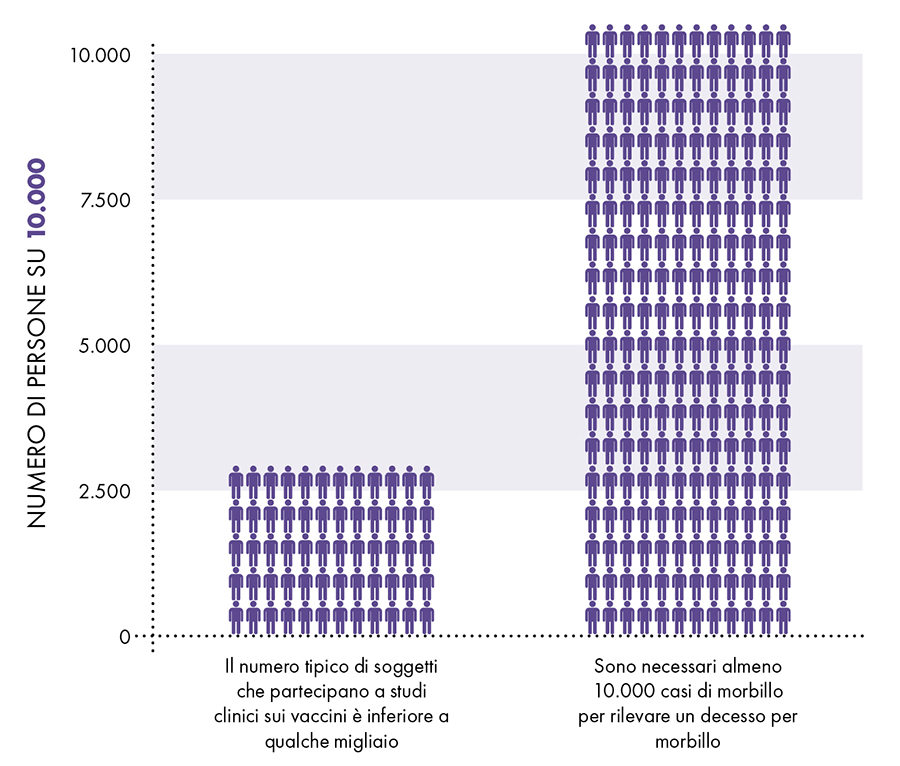 Figure 2: There are not enough subjects in clinical trials to show that the measles vaccine presents less risk than the measles vaccine.
Figure 2: There are not enough subjects in clinical trials to show that the measles vaccine presents less risk than the measles vaccine.
5. How accurate are epidemiological studies on the MMR vaccine?
Epidemiological studies are hampered by chance effects and possible confounders, i.e. additional factors that could affect the groups studied. For example, there is a well-known Danish study from 2002, published in the New England Journal of Medicine, which involved approximately 537.000 children and which looked for an association between the MMR vaccine and some adverse events.12 Raw data from the study were adjusted in an attempt to account for potential confounders, and the study found no association between the MMR vaccine and adverse events. However, as there is no evidence that the estimated confounders used to adjust the raw data were actually confounders, the study did not rule out the possibility that the MMR vaccine increases the risk of an adverse event leading to permanent injury by up to 77%. Consequently, the study did not rule out that such adverse events may occur up to four times more often than death from measles: 1 in 2.400 compared to 1 in 10.000 (Fig. 2 and Table 1). The range of possibilities found in the study, between adjusted data and raw data, makes the result inconclusive; even large epidemiological studies are not accurate enough to show that MMR vaccine causes fewer permanent deaths or lesions than measles.
Table 1: Statistical analysis of an epidemiological study with over half a million people.RR = relative risk CI = Confidence Interval Study-related adjusted RR = 0,92 (95% CI, 0,68 to 1,24) Unchanged RR recorded in the study (293 / 1.647.504) ÷ (53 / 482.360) = 1,45 (95% CI, 1,21 to 1,77) Potential RR = 1,77 Risk of the unvaccinated group recorded in the study = 53 out of 97.000 77% of 53 of 97.999 = 1 of 2.400 additional risk in the MMR vaccinated group |
6. Is the MMR vaccine safer than measles?
MMR vaccine has not been shown to be safer than measles. The vaccine package insert raises concerns about safety testing for cancer, genetic mutations and impaired fertility. Although VAERS tracks some adverse events, it is too inaccurate to measure against measles risk. Clinical studies are unable to detect less common adverse reactions and epidemiological studies are limited by case effects and possible confounders. Studies on the safety of the MMR vaccine are particularly lacking in statistical power. A review of more than 60 MMR vaccine studies conducted for the Cochrane Library states that: "The design and reporting of safety outcomes in MMR vaccine studies, both pre- and post-marketing, are largely inadequate.".13 Because the permanent (hangover) sequelae of measles, especially in individuals with normal vitamin A levels, are so rare,3 the level of accuracy of the available research studies is insufficient to show that the vaccine causes fewer permanent deaths or lesions than measles.
References
- Centers for Disease Control and Prevention. Washington, DC: US Department of Health and Human Services. Vaccines and immunizations: MMR vaccine side effects. [updated 2017 May 8; cited 2017 Jun 21]. https://www.cdc.gov/vaccines/vac-gen/side-effects.htm#mmr.
- Vestergaard M, Hviid A, Madsen KM, Wohlfahrt J, Thorsen P, Schendel D, Melbye M, Olsen J. MMR vaccination and febrile seizures: evaluation of susceptible subgroups and long-term prognosis. JAMA. 2004 Jul 21; 292 (3): 356.
- Physicians for Informed Consent. Newport Beach (CA): Physicians for Informed Consent. Measles - disease information statement (DIS); updated 2019 Sep. https: // www. physiciansforinformedconsent.org/measles/dis.
- World Health Organization. Measles vaccines: WHO position paper. Wkly Epidemiol Rec. 2009 Aug 28; 84 (35): 355.
- Merck. Whitehouse Station (NJ): Merck and Co., Inc. MMR II (measles, mumps, and rubella virus vaccine live); revised 2017 May [cited 2019 Aug 4]. https://www.merck.com/product/usa/pi_circulars/m/mmr_ii/mmr_ii_pi.pdf.
- Centers for Disease Control and Prevention. Washington, DC: US Department of Health and Human Services. CDC wonder: about the Vaccine Adverse Event Reporting System (VAERS); [cited 2017 Jun 21]. https://wonder.cdc.gov/vaers.html. Query for death and permanent disability involving all measles-containing vaccines, 2011-2015.
- Centers for Disease Control and Prevention. Manual for the surveillance of vaccine-preventable diseases. 5th ed. Miller ER, Haber P, Hibbs B, Broder K. Chapter 21: surveillance for adverse events following immunization using the Vaccine Adverse Event Reporting System (VAERS). Atlanta: Centers for Disease Control and Prevention; 2011. 1,2,8.
- Vaccine Adverse Event Reporting System. Washington, DC: US Department of Health and Human Services. Guide to interpreting VAERS data; [cited 2017 Jun 21]. https://vaers.hhs.gov/data/dataguide.html.
- Kessler DA. Introducing MEDWatch. A new approach to reporting medication and device adverse effects and product problems. JAMA. 1993 Jun 2; 269 (21): 2765-8
- Doshi P. The unofficial vaccine educators: are CDC funded non-profits sufficiently independent? [letter]. BMJ. 2017 Nov 7 [cited 2017 Nov 20]; 359: j5104. http://www.bmj.com/content/359/bmj. j5104 / rr-13.
- Centers for Disease Control and Prevention. Washington, DC: US Department of Health and Human Services. CDC wonder: about the Vaccine Adverse Event Reporting System (VAERS); [cited 2017 Jun 21]. https://wonder.cdc.gov/vaers.html.
- Madsen KM, Hviid A, Vestergaard M, Schendel D, WohlFahrt J, Thorsen P, Olsen J, Melbye M. A population-based study of measles, mumps, and rubella vaccination and autism. N Engl J Med. 2002 Nov 7; 347 (19): 1477,1480.
- Demicheli V, Rivetti A, Debalini MG, Di Pietrantonj C. Vaccines for measles, mumps and rubella in children. Cochrane Database of Syst Rev. 2012 Feb 15; (2).
Article translated by Physicians for Informed Consent

