Covid-19 Vaccine Obligations: 20 Scientific Facts That Challenge Them

HYPOTHESIS: COVID-19 vaccines significantly reduce the spread of COVID-19, so high universal vaccination rates will prevent outbreaks and end the pandemic |
DONE #1: a study on a COVID-19 outbreak in July 2021 published in Eurosurveillance found that "All transmissions between patients and staff occurred between masked and vaccinated individuals, as occurred in an outbreak in Finland". The authors state that the study "challenges the hypothesis that high universal vaccination rates lead to herd immunity and prevent outbreaks of COVID-19".1
DONE #2: a Centers for Disease Control and Prevention study of another COVID-19 outbreak in July 2021 found that 74 percent of cases were fully vaccinated.2
DONE #3: A Harvard study that analyzed COVID-19 cases in 68 countries and 2.947 US counties found no "no significant signs of a decrease in COVID-19 cases in the presence of higher percentages of the fully vaccinated population ".3
HYPOTHESIS: COVID-19 vaccines prevent death from COVID-19 |
DONE #4: Clinical trials have observed tens of thousands of subjects and are the only ones that included a control group and in which all subjects were monitored and tested for COVID-19 regardless of vaccination status. However, these studies did not find a sufficient number of deaths from COVID-19 to measure a significant difference in mortality between vaccinated and unvaccinated patients.4-7 The U.S. Food and Drug Administration (FDA) states that: "More individuals at high risk of COVID-19 and higher attack rates would be needed to confirm the effectiveness of the vaccine against mortality.".4-7
DONE #5: A study of a COVID-19 outbreak in July 2021 published in Eurosurveillance noted that 100% of serious, critical and fatal cases of COVID-19 occurred in vaccinated individuals.1
DONE #6: CDC data shows that mass vaccination with the COVID-19 vaccine did not have a measurable impact on the death rate from COVID-19 in the United States. In the nine months leading up to the introduction of mass vaccination (April 2020 to December 2020), there were approximately 356.000 deaths from COVID-19, or 39.500 deaths per month - a death rate of 0,120 per 1.000 people. In the nine months following the introduction of mass vaccination (January 2021 to September 2021), there were 342.000 deaths from COVID-19 or 38.000 deaths per month - a death rate of 0,115 per 1.000 people. In the following five months (from October 2021 to February 2022), there were another 249.000 deaths from COVID-19, or 49.800 deaths per month, with a death rate of 0,151 per 1.000 people.7
HYPOTHESIS: For children, COVID-19 vaccine injection is safer than SARS-CoV-2 infection. |
DONE #7: In the Pfizer clinical study, there were no cases of severe COVID-19 in children who did not receive the vaccine.8-9 In contrast, for children aged 5 years or older, Pfizer's clinical study of the COVID-19 vaccine found that the vaccine causes severe (grade 3) systemic reactions that include fever above 39 ° C, vomiting that requires hydration to intravenous route, 24-hour diarrhea and severe fatigue, severe headache, severe muscle aches or severe joint pain that prevent daily activity.9-12
DONE #8: In the clinical study, 1 in 59 to 1 in 143 vaccinated children aged 5 to 11 years experienced severe systemic reactions within seven days of the second dose. In the vaccinated group, 3 to 8 cases of serious systemic reactions were observed for every 10 non-serious COVID-19 cases in the unvaccinated group.9
DONE #9: In the clinical study, 1 in 9 vaccinated adolescents between the ages of 12 and 15 experienced severe systemic reactions within seven days of receiving the second dose. Severe systemic reactions observed in the vaccinated group were 7 times greater than non-serious cases of COVID-19 in the unvaccinated group.10-12
DONE #10: The clinical study also found that 1 in approximately 1.100 vaccinated children aged 12 to 15 had a grade 4 systemic reaction (fever above 39 ° C) after the first dose that required an emergency room visit and withdrawal from the study.10-13
HYPOTHESIS: The clinical trial of the COVID-19 vaccine was large enough to demonstrate safety in children.
|
FACT # 11: Pfizer's clinical study did not have sufficient statistical power to demonstrate that the vaccine is safe in children under the age of 18, as the study did not include enough subjects to establish safety (i.e., the clinical study included only about 2.600 vaccinated children between the ages of 5 and 15).9-14 In comparison, deaths due to COVID-19 are known to be rare in children. As of November 3, 2021, the chance that a 17-year-old or younger will contract SARS-CoV-2 and die from COVID-19 was 1 in 126.000 or 0,0008%.15
The clinical trial of the COVID-19 vaccine is inadequate to demonstrate safety in children
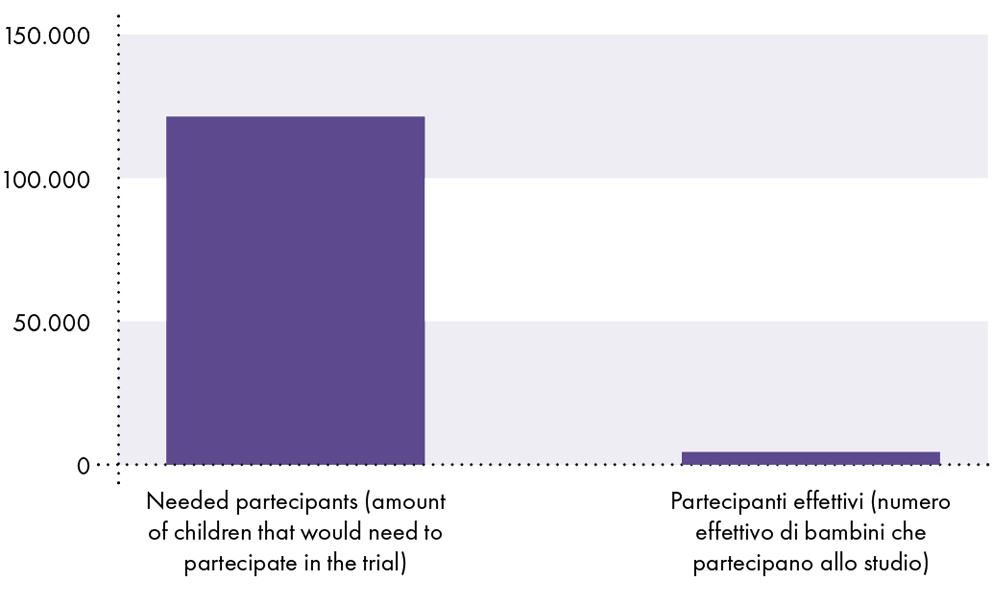
Because the likelihood of a child contracting SARS-CoV-2 and dying from COVID-19 is 0,0008%, or 1 in 126.000, at least 126.000 children are needed to detect a death from COVID-19. Therefore, there must be at least 126.000 vaccinated participants enrolled in the clinical trial to compare the risk of death from COVID-19 with the risk of death from vaccines. However, only about 2.600 vaccinated children participated in the clinical study.
HYPOTHESIS: COVID-19 vaccines are known to have no long-term side effects.
|
FACT # 12: Since all clinical trial subjects were only observed for two to six months, the long-term safety of COVID-19 vaccines for any age group is unknown. According to the FDA, there is currently insufficient data to draw conclusions on the safety of Pfizer, Moderna, and Johnson & Johnson vaccines in subpopulations such as pregnant and lactating individuals and immunocompromised individuals.4-8-16 For Pfizer, the vaccine "has not been evaluated for potential to cause carcinogenicity, genotoxicity or impairment of male fertility".17
FACT # 13: Safety surveillance reports identified serious risks of myocarditis and pericarditis in individuals under the age of 40, within seven days of vaccination. In 16- or 17-year-old boys, the FDA has reported an excess risk of myocarditis or pericarditis of 1 in 5.000 after the second dose of Pfizer COVID-19.18 vaccine AND in boys 12 to 17 years of age, again after a second dose of the Pfizer COVID-19 vaccine, a Hong Kong study found an excess risk of myocarditis or pericarditis of 1 in 2.700.19
HYPOTHESIS: The boosters will solve the problem of the drop in vaccine immunity.
|
FACT # 14: Clinical studies have found that vaccine immunity decreases significantly over a short period of time. For example, the effectiveness of the Pfizer vaccine decreased from 8% to 18% in just six months and the effectiveness of the Johnson & Johnson vaccine decreased from 25% to 29% in just six months.20-21 Furthermore, the efficacy measured in clinical trials was against the original Wuhan strain, not against the new variants.
FACT # 15: In clinical trials, a third dose of Pfizer or Moderna vaccine or a second dose of Johnson & Johnson vaccine was not evaluated for efficacy against the disease, but rather antibody counts were observed in a small number of vaccinated subjects for just one month.18-21-22
HYPOTHESIS: There are no known effective treatment or prevention options for COVID-19, except for vaccines.
|
FACT # 16Treatments for COVID-19 have improved significantly since the start of the pandemic in early 2020, resulting in improved survival rates in hospitalized cases.23-24 In fact, for people who do not live in a nursing home, the overall survival rate of COVID-19 is 99,8% in the United States and 99,999% for children in particular.25-26
FACT # 17: Hundreds of studies have looked at the effectiveness of various treatments, the most studied of which are ivermectin, vitamin D, hydroxychloroquine (HCQ) and monoclonal antibodies.27-30 These treatments may also be useful for prophylaxis (i.e., pre-exposure or post-exposure prevention of symptomatic COVID-19 infections).31-35
HYPOTHESIS: People who have previously been infected with SARS-CoV-2 need to get vaccinated because natural immunity is insufficient. |
FACT # 18Previous SARS-CoV-2 infection is shown to be more effective in preventing SARS-CoV-2 infection than COVID-19 vaccines. Johnson & Johnson's COVID-19 vaccine clinical trial included over 2.000 subjects who had contracted SARS-CoV-2 prior to the study. The study, which consistently tested unvaccinated and vaccinated people, recorded the incidence of COVID-19 in the unvaccinated group at least 28 days after vaccinating the other study subjects. The incidence of COVID-19 in the unvaccinated group with prior SARS-CoV-2 infection was 0,1% (2 / 2.021), while the incidence of COVID-19 in the vaccinated subjects was 0,59 % (113 / 19.306). These data suggest that COVID-19 cases in vaccinated subjects are 6 times higher than in unvaccinated subjects previously infected with SARS-CoV-2.36
FACT # 19Data from the Johnson & Johnson clinical study also indicates that an unvaccinated person previously infected with SARS-CoV-2 has a 99,9% chance of being protected from a new infection. It should be noted that as of July 1, 2021, 177,4 million SARS-CoV-2 infections were recorded in the United States, equal to 53,8% of the US population.26-36
HYPOTHESIS: Mandatory vaccines have been shown to create a safer environment. |
FACT # 20: Infection and transmission of SARS-CoV-2 occur at high rates in fully vaccinated populations, and a significant percentage of severe, critical and fatal cases of COVID-19 occur in fully vaccinated individuals. Data from the CDC shows that mass vaccination with COVID-19 vaccines did not have a measurable impact on COVID-19 mortality in the United States. Additionally, data from short-term clinical trials indicate that 1 in 6 to 1 in 9 people aged 12 to 55 who receive the mRNA-based COVID-19 vaccine suffer from severe (Grade 3) systemic reactions. ) and no long-term safety studies have been conducted.13-37 Therefore, scientific data show that compulsory vaccination cannot create a safer environment.
References
- Shitrit P, Zuckerman NS, Mor O, Gottesman BS, Chowers M. Nosocomial outbreak caused by the SARS-CoV-2 Delta variant in a highly vaccinated population, Israel, July 2021. Euro Surveill. 2021 Sep; 26 (39). https://pubmed.ncbi.nlm.nih.gov/34596015/.
- Brown CM, Vostok J, Johnson H, Burns M, Gharpure R, Sami S, Sabo RT, Hall N, Foreman A, Schubert PL, Gallagher GR, Fink T, Madoff LC, Gabriel SB, MacInnis B, Park DJ, Siddle KJ , Harik V, Arvidson D, Brock-Fisher T, Dunn M, Kearns A, Laney AS. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings — Barnstable County, Massachusetts, July 2021. MMWR Morb Mortal Wkly Rep. 2021 Aug 6; 70 (31): 1059-62. https://www.cdc.gov/mmwr/volumes/70/wr/mm7031e2.htm?s_cid=mm7031e2_w.
- Subramanian SV, Kumar A. Increases in COVID-19 are unrelated to levels of vaccination across 68 countries and 2947 counties in the United States. Eur J Epidemiol. 2021 Sep 30: 1-4. https://pubmed.ncbi.nlm.nih.gov/34591202/.
- US Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Modern COVID-19 vaccine. Vaccines and Related Biological Products Advisory Committee Meeting: December 17, 2020. https://www.fda.gov/media/144434/download.
- US Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Janssen Ad26.COV2.S vaccine for the prevention of COVID-19. Vaccines and Related Biological Products Advisory Committee Meeting: February 26, 2021. Table 22: vaccine efficacy of first occurrence of moderate to severe / critical and severe / critical COVID-19 including non-centrally confirmed cases with onset at least 14 or at least 28 days after vaccination, by country of participation, per-protocol set, study 3001; 37. https://www.fda.gov/media/146217/download.
- US Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Pfizer-BioNTech COVID-19 vaccine. Vaccines and Related Biological Products Advisory Committee Meeting: December 10, 2020. https://www.fda.gov/media/144245/download.
- Centers for Disease Control and Prevention. Washington, DC: US Department of Health and Human Services. COVID data tracker: trends in number of COVID-19 cases and deaths in the US reported to CDC, by state / territory; [cited 2022 Apr 2]. https://covid.cdc.gov/covid-data-tracker/#trends_totaldeaths.
- US Food and Drug Administration, Center for Biologics Evaluation and Research (CBER) Office of Vaccines Research and Review (OVRR). Washington, DC: US Department of Health and Human Services. Emergency use authorization (EUA) amendment for an unapproved product: review memorandum; 2021 Apr 9: 23, 39. https://www.fda.gov/media/148542/download.
- US Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: EUA amendment request for Pfizer-BioNTech COVID-19 vaccine for use in children 5 through 11 years of age. Vaccines and Related Biological Products Advisory Committee Meeting: October 26, 2021. https://www.fda.gov/media/153447/download.
- Wallace M. Grading of recommendations, assessment, development, and evaluation (GRADE): Pfizer-BioNTech COVID-19 Vaccine. COVID-19 Vaccines Work Group of the Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention. 2021 May 12:24, 25. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-05-12/03-COVID-Wallace-508.pdf.
- Centers for Disease Control and Prevention. Washington, DC: US Department of Health and Human Services. Grading of recommendations, assessment, development, and evaluation (GRADE): Pfizer-BioNTech COVID-19 vaccine for persons aged 12-15 years; [cited 2021 May 14]. https://www.cdc.gov/vaccines/acip/recs/grade/covid-19-pfizer-biontech-vaccine-12-15-years.html#table03d.
- Pfizer. New York (NY): Pfizer Inc. Fact sheet for healthcare providers administering vaccine (vaccination providers); revised 2022 Jan 3. Table 11: vaccine efficacy - first COVID-19 occurrence from 7 days after dose 2: without evidence of infection and with or without evidence of infection prior to 7 days after dose 2 - blinded placebo-controlled follow-up period , adolescents 12 through 15 years of age evaluable efficacy (7 days) population; 48. https://www.fda.gov/media/153713/download.
- Physicians for Informed Consent. Pfizer COVID-19 Vaccine: Short-Term Efficacy & Safety Data. Dec 2021. https://www.physiciansforinformedconsent.org/COVID-19-vaccines.
- Pfizer. New York (NY): Pfizer Inc. Fact sheet for healthcare providers administering vaccine (vaccination providers); revised 2022 Jan 3:48. https://www.fda.gov/media/153713/download.
- Centers for Disease Control and Prevention. Washington, DC: US Department of Health and Human Services. Weekly updates by select demographic and geographic characteristics: provisional death counts for coronavirus disease (COVID-19); [cited 2021 Nov 3]. https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm#AgeAndSex.
- US Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Janssen Ad26.COV2.S vaccine for the prevention of COVID-19. Vaccines and Related Biological Products Advisory Committee Meeting: February 26, 2021. https://www.fda.gov/media/146217/download.
- Pfizer. New York (NY): Pfizer Inc. Comirnaty (COVID-19 vaccine, mRNA) suspension for injection, for intramuscular use; revised 2021 Dec. https://www.fda.gov/media/151707/download.
- US Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Application for licensure of a booster dose for Comirnaty (COVID-19 Vaccine, mRNA). Vaccines and Related Biological Products Advisory Committee Meeting: September 17, 2021. https://www.fda.gov/media/152176/download.
- Chua GT, Kwan MYW, Chui CSL, Smith RD, Cheung EC, Tian T, Leung MTY, Tsao SSL, Kan E, Ng WKC, Man Chan VC, Tai SM, Yu TC, Lee KP, Wong JSC, Lin YK, Shek CC, Leung ASY, Chow CK, Li KW, Ma J, Fung WY, Lee D, Ng MY, Wong WHS, Tsang HW, Kwok J, Leung D, Chung KL, Chow CB, Chan GCF, Leung WH, To KKW, Yuen KY, Lau YL, Wong ICK, Ip P. Epidemiology of acute myocarditis / pericarditis in Hong Kong adolescents following Comirnaty vaccination. Clin Infect Dis. 2021 Nov 28: ciab989. https://pubmed.ncbi.nlm.nih.gov/34849657.
- Thomas SJ, Moreira ED Jr, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Polack FP, Zerbini C, Bailey R, Swanson KA, Xu X, Roychoudhury S, Koury K, Bouguermouh S, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Yang Q, Liberator P, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Gruber WC, Jansen KU; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine through 6 months. N Engl J Med. 2021 Nov 4; 385 (19): 1761-73. https://pubmed.ncbi.nlm.nih.gov/34525277.
- US Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: EUA amendment request for a booster dose for the Janssen COVID-19 vaccine. Vaccines and Related Biological Products Advisory Committee Meeting: October 15, 2021. 21, 39. https://www.fda.gov/media/153037/download.
- US Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: EUA amendment request for a booster dose for the Moderna COVID-19 vaccine. Vaccines and Related Biological Products Advisory Committee Meeting: October 14, 2021. https://www.fda.gov/media/152991/download.
- Horwitz LI, Jones SA, Cerfolio RJ, Francois F, Greco J, Rudy B, Petrilli CM. Trends in COVID-19 risk-adjusted mortality rates. J Hosp Med. 2021 Feb; 16 (2): 90-2. https://www.journalofhospitalmedicine.com/jhospmed/article/230561/hospital-medicine/trends-covid-19-risk-adjusted-mortality-rates.
- Dennis JM, McGovern AP, Vollmer SJ, Mateen BA. Improving survival of critical care patients with coronavirus disease 2019 in England: a national cohort study, March to June 2020. Crit Care Med. 2021 Feb 1; 49 (2): 209-14. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7803441/.
- Ioannidis, JPA. Reconciling estimates of global spread and infection fatality rates of COVID- 19: an overview of systematic evaluations. Eur J Clin Invest. 2021; 51: e13554. https://onlinelibrary.wiley.com/doi/epdf/10.1111/eci.13554.
- Physicians for Informed Consent. COVID-19 - Disease Information Statement (DIS). Aug 2021. https://physiciansforinformedconsent.org/covid-19/.
- C19early. com. COVID-19 early treatment: real-time analysis of 1,298 studies; [cited 2022 Jan 11]. https://c19early.com/.
- Regeneron. Tarrytown, (NY): Regeneron Pharmaceuticals, Inc. Fact sheet for health care providers: emergency use authorization (EUA) of REGEN-COV (casirivimab and imdevimab); revised 2021 Dec. https://www.regeneron.com/downloads/treatment-covid19-eua-fact-sheet-for-hcp.pdf.
- Lilly. Indianapolis (IN): Eli Lilly and Company. Neutralizing antibodies for COVID-19; [cited 2022 Feb 9]. https://www.lilly.com/news/media/media-kits/bamlanivimab-covid19.
- GSK. London (UK): GlaxoSmithKline plc. GSK and Vir Biotechnology announce United States government agreement to purchase additional supply of sotrovimab, authorized for the early treatment of COVID-19; 2022 Jan 11 [cited 2022 Feb 9]. https://www.gsk.com/en-gb/media/press-releases/gsk-and-vir-biotechnology-announce-united-states-government-agreement-to-purchase-additional-supply-of-sotrovimab.
- C19early. com. COVID-19 studies: ivermectin; [cited 2022 Feb 12]. https://c19ivermectin.com.
- Bryant A, Lawrie TA, Dowswell T, Fordham EJ, Mitchell S, Hill SR, Tham TC. Ivermectin for prevention and treatment of COVID-19 infection: a systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am J Ther. 2021 Jun 21; 28 (4): e434-60. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8248252/.
- C19early. com. COVID-19 studies: vitamin D; [cited 2022 Feb 12]. https://c19vitamind.com.
- Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020 Jul; 32 (7): 1195-8. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7202265/.
- C19early. com. HCQ for COVID-19: real-time meta analysis of 303 studies; [cited 2022 Jan 10]. https://hcqmeta.com.
- US Food and Drug Administration, Vaccines and Related Biological Products Advisory Committee. FDA briefing document: Janssen Ad26.COV2.S vaccine for the prevention of COVID-19. Vaccines and Related Biological Products Advisory Committee Meeting: February 26, 2021. Table 14: vaccine efficacy of first occurrence of moderate to severe / critical COVID-19, including non-centrally confirmed cases, with onset at least 14 or at least 28 days after vaccination, by baseline SARS-CoV-2 status, per protocol set; 30. https://www.fda.gov/media/146217/download.
- El Sahly HM, Baden LR, Essink B, Doblecki-Lewis S, Martin JM, Anderson EJ, Campbell TB, Clark J, Jackson LA, Fichtenbaum CJ, Zervos M, Rankin B, Eder F, Feldman G, Kennelly C, Han- Conrad L, Levin M, Neuzil KM, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Polakowski L, Mascola JR, Ledgerwood JE, Graham BS, August A, Clouting H, Deng W, Han S, Leav B , Manzo D, Pajon R, Schödel F, Tomassini JE, Zhou H, Miller J; COVE Study Group. Efficacy of the mRNA-1273 SARS-CoV-2 vaccine at completion of blinded phase. N Engl J Med. 2021 Nov 4; 385 (19): 1774-85. Suppl appendix; 36-7. https://www.nejm.org/doi/suppl/10.1056/NEJMoa2113017/suppl_file/nejmoa2113017_appendix.pdf.
Article translated by Physicians for Informed Consent

