Can the Hepatitis B vaccine cause injury and/or death?

IMPORTANT NOTE: Corvelva invites you to get in-depth information by reading all the sections and links, as well as the manufacturer's product leaflets and technical data sheets, and to speak with one or more trusted professionals before deciding to vaccinate yourself or your child. This information is for informational purposes only and is not intended as medical advice.
The problem of multiple vaccines (click to open)
The problem of multiple vaccines

The current vaccination calendars, especially for the pediatric age, provide for the administration of multiple antigens and vaccines in a single session, favoring comfort at the expense of safety. In order to be able to make a specific speech on the safety of vaccines, we must necessarily take into consideration the complexity of the phenomenon, advising all readers to adequately inform themselves on all aspects of vaccination, pros and cons.
Dr. Russell Blaylock, clinical assistant professor of neurosurgery at the University of Mississippi Medical Center, has studied "toxic synergy" for years and was able to observe that when two weakly toxic pesticides, where neither is able to cause Parkinson's syndrome in experimental animals, are combined with each other, can cause the disease even quickly and compares this phenomenon to that of multiple vaccines administered simultaneously: "Vaccinations, if too numerous and too close together, behave like a disease chronic".(a). Others Two studies have confirmed that sudden infant death can occur after inoculation of multiple vaccines in a single administration.(bc)
A study published in Human and Experimental Toxicology showed that countries that prescribe more vaccines in children tend to have higher infant mortality rates.(D) For example, in the United States, where children receive 26 vaccines, more than 6 children per 1000 live births die, while in Sweden and Japan, where 12 pediatric vaccines are administered, 3 deaths are reported for every 1000 live births. In the aforementioned study, the link between vaccines and SIDS is also considered.
From a Swiss study published in 2005 in the European Journal of Pediatrics(E) it results that, regarding the effects on preterm infants, the incidence of recurrent or increased apnea and bradycardia after administration of hexavalent vaccines is 13%. That same year, the same journal published a German study that had examined sudden infant deaths after hexavalent. The authors write: «These results, based on spontaneous reports, do not prove a causal relationship between vaccination and sudden infant death, but constitute a signal regarding one of the two available hexavalents; signal that should lead to intensify surveillance of sudden infant deaths after vaccination".(f)
In 2006, it was published in the medical journal Vaccine(g) the letter from a team of researchers from the University of Munich which reported «six cases of sudden infant death after hexavalent vaccination.. All found dead without explanation 1-2 days after vaccination». They had been classified as typical cases of sudden infant death but the autoptic verification had revealed neuropathological and histological abnormalities and all the children showed a significant cerebral edema which made them an exception compared to the other SIDS cases (Sudden Infant Death Syndrome). The researchers wrote that “Before the introduction of the hexavalent vaccine (in the years 1994-2000), we had observed the case of only one in 198 children with sudden infant death who died soon after the DTP vaccination. But between 2001 and 2004 they had identified five similar cases out of 74 with SIDS. That would indicate a thirteen-fold increase."
Also in 2006 on Virchows Archive(H), the team from the Institute of Pathology of the University of Milan wrote: «Experts from the European Agency for the Evaluation of Medical Products have analyzed the possibility that there could be a link between hexavalent vaccines and some cases of death. Participants included pathologists with experience in vaccines and sudden infant death syndrome who conducted the autopsies. But, as far as we know, little attention was paid to examination of the brainstem and blood heart on serial sections and there was no possibility of establishing a trigger role of the vaccine for these deaths. Here we report the case of a 3-month-old girl who died suddenly after hexavalent vaccination. Examination of the brain stem on serial sections revealed bilateral arcuate nucleus hypoplasia. The conduction system of the heart had persistent fetal dispersion and degeneration. This case offers a unique understanding of the possible role of the hexavalent vaccine in triggering a lethal consequence in a vulnerable child. Any case of sudden and unexpected death that occurs soon after birth or in early childhood, especially if following a vaccination, should always undergo a full necropsy, according to guidelines.
References
- Blaylock R, "Vaccinations: the hidden dangers", The Blaylock Wellness Report, May 2004, pp.1-9
- Ottaviani G. et al., "Sudden infant death syndrome (SIDS) shortly after hexavalent vaccination: another pathology in suspected SIDS?", Virchows Archiv., 2006, 448, pp. 100-104.
- Zinka B. et al., "Unexplained cases of sudden infant death shortly after hexavalent vaccination", Vaccine, July 2006, 24 (31-32), pp. 5779-5780.
- Miller NZ et al1. , "Infant mortality rates regressed against number of vaccine doses routinely given: there is a biochemical or synergistic toxicity?", Hum. Exp. Toxicol., May 2011.
- https://pubmed.ncbi.nlm.nih.gov/15843978/
- https://pubmed.ncbi.nlm.nih.gov/15602672/
- https://pubmed.ncbi.nlm.nih.gov/15908063/
- https://pubmed.ncbi.nlm.nih.gov/16231176/
The aluminum problem (click to open)
Aluminum in vaccines: what parents need to know

1. What is aluminum?
Aluminum is a silvery-white light metal, malleable and resistant. These qualities make it useful in numerous industries and products, including machinery, construction, warehouses, cookware, kitchen utensils, textiles, dyes and cosmetics. Aluminum is also the most abundant metal in the earth's crust, and virtually all of the aluminum in the environment is found in the soil. However, aluminum is not found naturally in significant quantities in living organisms (such as plants and animals) and has no known biological function. Over the past century, the use of aluminum in some products has led to increased human exposure. The major sources of exposure are aluminum-containing foods (e.g., baking powder, processed foods, baby formulas, etc.), medical products (e.g., antiperspirants, antacids, etc.), allergy injections, and vaccines .1-3
2. Why is aluminum present in vaccines?
Some vaccines use aluminum compounds (aluminum hydroxide and aluminum phosphate) as adjuvants, which are ingredients that increase the immune response to an antigen (foreign substance).4-5 The US Food and Drug Administration (FDA) says that if some vaccines did not include aluminum, the immune response they trigger could decrease.6
3. Which vaccines contain aluminum?
The following vaccines contain aluminum and are given to infants, children and adolescents (Fig. 1):
- Hepatitis B (HepB)
- hexavalent
- Diphtheria, tetanus and pertussis (DTaP and Tdap)
- Haemophilus influenzae type b (PedvaxHIB)
- Pneumococcus (PCV)
- Hepatitis A (HepA)
- Papillomavirus virus (HPV)
- Meningococcus B (MenB)
Figure 1: Up to 18 doses of aluminum-containing vaccines are administered from birth to age 227-8
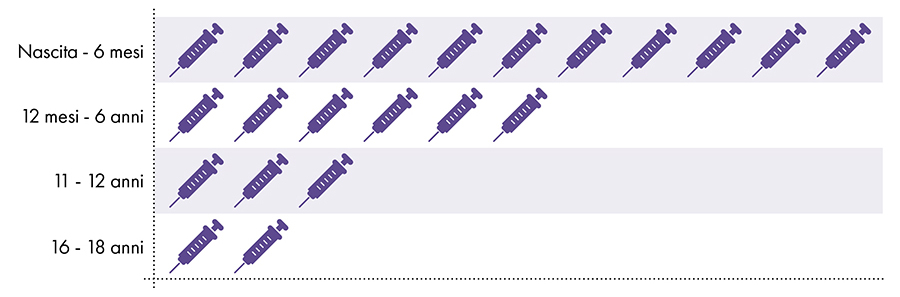
4. Is exposure to aluminum safe?
The FDA has considered aluminum generally recognized as safe (GRAS) since 1975.9 However, prior to 1990, there was no technology to accurately detect small amounts of aluminum administered to subjects in scientific studies.10 Consequently, the amount of aluminum that could be absorbed before the onset of adverse effects was not known.
Since the 1990s, thanks to technological advances, it has been observed that the small amounts of aluminum that remain in the human body interfere with a number of cellular and metabolic processes in the nervous system and tissues of other parts of the body.1-10-11 The greatest negative effects of aluminum have been observed in the nervous system and range from impaired motor skills to encephalopathy (altered mental status, personality changes, thinking difficulties, memory loss, seizures, coma and more).2-12
The United States Department of Health and Human Services (HHS) recognizes aluminum as a known neurotoxin.2 Additionally, the FDA has warned about the risks of aluminum toxicity in infants and children.13
FEDERAL REGISTER: The daily newspaper of the United States government"Even full-term infants with normal kidney function may be at risk due to rapid growth and immaturity of the brain and skeleton, as well as immaturity of the blood-brain barrier. Up to the age of 1 or 2, infants have a lower glomerular filtration rate than adults, which affects their kidney function. The agency fears that young children and those with immature kidney function are at increased risk of aluminum exposure. " |
5. How much oral aluminum is not safe?
In 2008, the Agency for Toxic Substances and Disease Registry (ATSDR), a division of HHS, used studies on the neurotoxic effects of aluminum to determine that no more than 1 milligram (1.000 micrograms) of aluminum per kilogram of body weight per day to avoid the negative effects of aluminum.2
6. How much aluminum injected is not safe?
To determine the amount of aluminum that can be safely injected it is necessary to convert the oral aluminum limit of the ATSDR. The ATSDR limit for oral aluminum (1.000 micrograms of aluminum per kilogram of body weight per day) is based on 0,1% of the oral aluminum that is absorbed into the bloodstream, as the digestive tract blocks almost all of the oral aluminum .2 In contrast, aluminum injected intramuscularly bypasses the digestive tract and 100% of the aluminum can be absorbed into the bloodstream over time (i.e., the proportion of aluminum absorbed is 1.000 times greater). To account for these different amounts of absorption, the oral aluminum limit of the ATSDR must be divided by 1000. This conversion leads to an ATSDR-derived blood aluminum limit of 1 microgram of aluminum (0,1% of 1.000 micrograms) per kilogram of body weight per day. Consequently, to avoid the neurotoxic effects of aluminum, no more than 1 microgram of aluminum per kilogram of body weight should enter the bloodstream on a daily basis. Figure 3 shows the ATSDR-derived blood aluminum limit for infants of various ages based on their weight.
7. How much aluminum is there in vaccines?
The amount of aluminum in vaccines varies.16 In 1968, the US federal government set the limit for the amount of aluminum in vaccines at 850 micrograms per dose, based on the amount of aluminum needed to make some vaccines effective.6-17 Consequently, the amount of aluminum in aluminum-containing infant vaccines ranges from 125 to 850 micrograms per dose. Figure 4 shows the aluminum content of one dose of various vaccines given to children.
8. Have any studies compared the amount of aluminum in vaccines with the limit derived from the Agency for Toxic Substances and Disease Registry (ATSDR)?
In 2011, a study was published that aimed to compare the amount of aluminum in vaccines with the blood flow limit set by the ATSDR.18 However, this study incorrectly based its calculations on 0,78% oral aluminum absorbed into the bloodstream, rather than the 0,1% value used by the ATSDR in its calculations.19-20 As a result, the 2011 study hypothesized that nearly 8 times (0,78% / 0,1%) aluminum can safely enter the bloodstream, and this has led to an incorrect conclusion.
9. Is aluminum exposure from vaccines safe?
Vaccines are injected intramuscularly, and the rate at which aluminum from vaccines migrates from human muscle into the bloodstream is unknown. Animal studies suggest that aluminum from vaccines can take anywhere from a couple of months to more than a year to enter the bloodstream, due to multiple variables.21-23 Since cumulative aluminum exposure from vaccines in children under one year of age exceeds the daily limit set by the ATSDR by several hundred (Fig. 3 and 4), the limit would still be exceeded if aluminum from vaccines entered the blood flow over the course of about a year. In addition, studies have shown that aluminum from vaccines is absorbed by immune cells and reaches parts of the body far from the injection site, including the brain.24
The extent of the adverse effects of aluminum in vaccines is not known, as safety studies have not been conducted comparing a population vaccinated with aluminum-containing vaccines with a population not vaccinated with such vaccines.
Aluminum limitation of blood flow derived from the ATSDR2-14-15
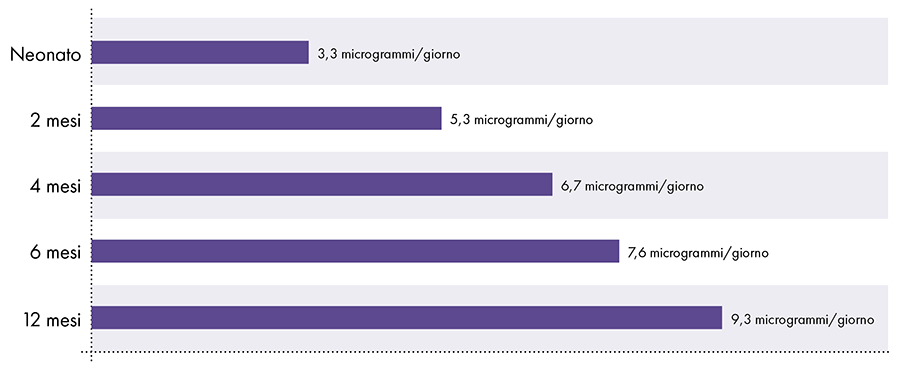 Figure 3: This graph shows the aluminum limit for children of various ages, as derived from the Toxic Substances and Disease Registry, a division of the United States Department of Health and Human Services. The limit indicates that no more than 1 microgram of aluminum per kilogram of body weight should enter the bloodstream on a daily basis to avoid the neurotoxic effects of aluminum.
Figure 3: This graph shows the aluminum limit for children of various ages, as derived from the Toxic Substances and Disease Registry, a division of the United States Department of Health and Human Services. The limit indicates that no more than 1 microgram of aluminum per kilogram of body weight should enter the bloodstream on a daily basis to avoid the neurotoxic effects of aluminum.
Amount of aluminum in vaccines
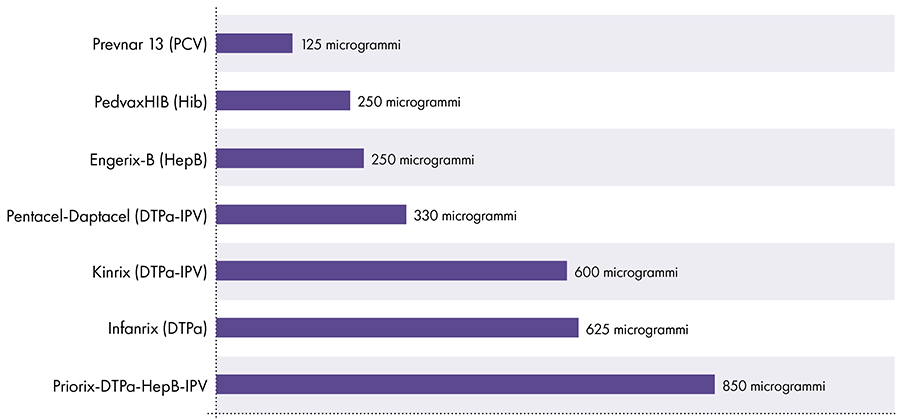
References
- American Academy of Pediatrics, Committee on Nutrition. Aluminum toxicity in infants and children. Pediatrics. 1996 Mar; 97 (3): 413.
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological profile for aluminum. Washington, DC: US Department of Health and Human Services; 2008.3, 13-24, 145, 171-7, 208.
- Yokel RA. Aluminum in food — the nature and contribution of food additives. In: El-Samragy Y, editor. Food additive. Rijeka (Croatia): InTech; 2012. 203-28.
- Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminum. Nat Rev Immunol. 2009 Apr; 9 (4): 287.
- Volk VK, Bunney WE. Diphtheria immunization with fluid toxoid and alum-precipitated toxoid. Am J Public Health Nations Health. 1942 Jul; 32 (7): 690-9.
- Baylor NW, Egan W, Richman P. Aluminum salts in vaccines — US perspective. Vaccine. 2002 May 31; 20 Suppl 3: S18-22.
- US Food and Drug Administration. Silver Spring (MD): US Food and Drug Administration. Vaccines licensed for use in the United States; [updated 2018 Feb 14; cited 2018 Feb 27]. https://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/Ucm093833.htm.
- Centers for Disease Control and Prevention. Washington, DC: US Department of Health and Human Services. Recommended immunization schedule for children and adolescents aged 18 years or younger, United States, 2018. https://www.cdc.gov/vaccines/schedules/downloads/child/0-18yrs-child-combined-schedule.pdf.
- US Food and Drug Administration. Silver Spring (MD): US Food and Drug Administration. SCOGS (Select Committee on GRAS Substances); [cited 2018 Aug 16]. https://www.accessdata.fda.gov/scripts/fdcc/?set=SCOGS.
- Priest ND. The biological behavior and bioavailability of aluminum in man, with special reference to studies employing aluminum-26 as a tracer: review and study update. J Environ Monit. 2004; 6: 376,392.
- Poole RL, Pieroni KP, Gaskari S, Dixon TK, Park KT, Kerner JA. Aluminum in pediatric parenteral nutrition products: measured versus labeled content. J Pediatr Pharmacol Ther. 2011; 16 (2): 92-7.
- Sedman A. Aluminum toxicity in childhood. Pediatr Nephrol. 1992 Jul; 6 (4): 383-93.
- US Food and Drug Administration, Department of Health and Human Services. Rules and regulations. Fed Regist. 2003 Jun; 68 (100): 34286.
- Centers for Disease Control and Prevention. Washington, DC: US Department of Health and Human Services. National Center for Health Statistics: Data table for boys length-for-age and weight-for-age charts; [cited 2019 April 2]. https://www.cdc.gov/growthcharts/who/boys_length_weight.htm.
- Centers for Disease Control and Prevention. Washington, DC: US Department of Health and Human Services. National Center for Health Statistics: Data table for girls length-for-age and weight-for-age charts; [cited 2019 April 2]. https://www.cdc.gov/growthcharts/who/girls_length_weight.htm.
- US Food and Drug Administration, Department of Health and Human Services. Revision of the requirements for constituent materials. Final rule. Fed Regist. 2011 Apr 13; 76 (71): 20513-8.
- Office of the Federal Register, National Archives and Records Service, General Services Administration. Rules and regulations. Fed Regist. 1968 Jan; 33 (6): 369.
- Mitkus RJ, King DB, Hess MA, Forshee RA, Walderhaug MO. Updated aluminum pharmacokinetics following infant exposures through diet and vaccination. Vaccine. 2011 Nov 28; 29 (51): 9538-43.
- Miller S, Physicians for Informed Consent. Erratum in 'Updated aluminum pharmacokinetics following infant exposures through diet and vaccination.' In: ResearchGate. Berlin (Germany): ResearchGate GmbH; 2020 Mar 6 [cited 2020 Mar 6]. https://www.researchgate.net/publication/51718934_Updated_Aluminum_pharmacokinetics_following_infant_exposures_through_diet_and_vaccines/comments.
- Physicians for Informed Consent. Newport Beach (CA): Physicians for Informed Consent. Erratum in 'Updated aluminum pharmacokinetics following infant exposures through diet and vaccination'; [cited 2020 Mar 6]. https://physiciansforinformedconsent.org/mitkus-2011-erratum/.
- Flarend RE, Hem SL, White JL, Elmore D, Suckow MA, Rudy AC, Dandashli EA. In vivo absorption of aluminum-containing vaccine adjuvants using 26Al. Vaccine 1997 Aug-Sept; 15 (12-13): 1314-8.
- Verdier F, Burnett R, Michelet-Habchi C, Moretto P, Fievet-Groyne F, Sauzeat E. Aluminum assay and evaluation of the local reaction at several time points after intramuscular administration of aluminum containing vaccines in the Cynomolgus monkey. Vaccine. 2005 Feb 3; 23 (11): 1359-67.
- Weisser K, Göen T, Oduro JD, Wangorsch G, Hanschmann KO, Keller-Stanislawski B. Aluminum in plasma and tissues after intramuscular injection of adjuvanted human vaccines in rats. Arch Toxicol. 2019 Oct; 93 (10): 2787-96.
- Masson JD, Crépeaux G, Authier FJ, Exley C, Gherardi RK. Critical analysis of reference studies on the toxicokinetics of aluminum-based adjuvants. J Inorg Biochem. 2018 Apr; 181: 87-95.
Article translated by Physicians for Informed Consent
In 1981, the US Food and Drug Administration (FDA) authorized a plasma-derived hepatitis B vaccine that contained antigens taken from infected individuals. This vaccine was later withdrawn from the market because, like all vaccines produced from human blood, it was capable of transmitting unwanted and potentially dangerous viruses. In 1986, the first of a series of genetically modified (recombinant DNA) vaccines was authorized.
Numerous studies have investigated the probability that those who received the plasma-derived vaccine could also have received unwanted viruses, especially HIV, the precursor to AIDS.(1-2) Furthermore, clinical studies to attest to the safety of the current hepatitis B vaccine were only carried out on 147 healthy children monitored for just 5 days after administration(3) This is not a large enough sample nor a long enough period to determine the true incidences of adverse events. The manufacturing companies themselves have admitted that "widespread use of the vaccine could lead to the emergence of adverse reactions not observed in clinical trials".(4)
Even adult subjects were monitored for only five days after vaccination and, despite this, systemic effects such as arthralgia, myalgia, paraesthesia, back and neck pain, lymphadenopathy, headache, fever, malaise, chills, vomiting were still reported. , diarrhea, abdominal pain, upper respiratory tract infections, earache and hypotension.(5)
Despite the official technical data sheets, and other documents that propagate(6) the hepatitis B vaccine, tend to minimize or deny serious adverse reactions, numerous studies published in medical and scientific journals around the world and reports forwarded to VAERS(7) confirm various pathologies as a consequence of vaccination. Some of these studies are summarized below.
Arthritis
In 1990, soon after the introduction of the hepatitis B vaccine, the British Medical Journal documented a link between the vaccine and polyarthritis, a painful inflammation of five or more joints.(8) The same year the Journal of Rheumatology published a paper on reactive arthritis after hepatitis B vaccination.(9)
In 1994, the British Journal of Rheumatology released data documenting rheumatoid arthritis after the vaccine(10) and the BMJ published three further reports confirming the link between the vaccine and reactive arthritis.(11-12) In 1995, two studies were published in the Scandinavian Journal of Rheumatology confirming cases of post-vaccination arthritis(13-14) and that same year the Irish Medical Journal documented the link with arthropathy.(15) In 1997, the British Journal of Rheumatology published two other studies documenting several cases of inflammatory polyarthritis after the vaccine(16-17) and in 1998 the Journal of Rheumatology again confirmed rheumatoid arthritis.(18) Also in that year, the French magazine Revue de Médecine Interne published a study on Still's disease with onset in adulthood - a rare and painful type of arthritis - after vaccination for hepatitis A and B.(19) In 1999, Rheumatology documented rheumatological disorders after the vaccine(20) and in 2000 the American College of Rheumatology published research in the peer-reviewed journal Arthritis & Rheumatology that documented Sjogren's syndrome - a rare form of chronic arthritis - after hepatitis B vaccination.(21)
Autoimmune and neurological diseases including multiple sclerosis
In 1983, the New England Journal of Medicine published a study demonstrating the onset of polyneuropathy - simultaneous malfunction of numerous nerves - after hepatitis B vaccination.(22) In 1988, the American Journal of Epidemiology reported multiple “neurological adverse events” after the vaccine including numerous cases of Guillain-Barré syndrome, lumbar radiculopathy, brachial plexus neuropathy, optic neuritis, and transverse myelitis.(23) The same year, the journal Archives of Internal Medicine documented myasthenia gravis - a severe chronic autoimmune neuromuscular disease - again after hepatitis B vaccination.(24)
In 1991 The Lancet published a report documenting central nervous system demyelination after the vaccine(25) and in 1992 Nephron released data linking vaccination to systemic lupus erythematosus, a chronic autoimmune disease that affects multiple organs.(26) Also in 1992, the journal Clinical Infectious Diseases published a study linking Evans syndrome - a rare autoimmune and blood disease with a high mortality rate - to the vaccine.(27) and the French magazine Thérapie published a study on "peripheral facial paralysis" again after administration of the drug.(28) Additionally, Infectious Disease News released a report stating numerous cases of neurological damage resembling multiple sclerosis(29) and in 1993 an article appeared in the Journal of Hepatology on transverse myelitis - inflammation of the spinal cord - after anti-hepatitis B vaccination.(30) That same year the French newspaper La Nouvelle Presse Médicale published data confirming post-vaccination "acute myelitis"(31) and Clinical Infectious Diseases documented “classical multiple sclerosis.”(32) In 1994, Archives of Pediatrics and Adolescent Medicine released data linking lupus to the vaccine(33) and the journal Acta Neurologica Scandinavica published a report on acute cerebellar ataxia - severe loss of balance and motor coordination - after the vaccination in question.(34)
In 1995, demyelination of the central nervous system was reported in the Journal of Neurology, Neurosurgery and Psychiatry(35) and in the American Journal of Neuroradiology myelitis. The authors of the latter study noted that adverse events of this nature may be under-reported because symptoms are late.(36) In 1996, both Nephron and the French journal Annales de Dermatologie et de Vénéréologie published studies attesting to the correlation between lupus erythematosus and the hepatitis B vaccine.(37-38) The same year the Journal of Hepatology published a report on the link with leukoencephalitis, inflammation of the white matter of the brain.(39) In 1996, the New England Journal of Medicine documented postvaccination cryoglobulinemia, a rare autoimmune disease that impairs circulation, causes bleeding and other problems.(40)
Vaccine-induced autoimmunity was certified in the Journal of Autoimmunity(41) and in 1997 the Indian Journal of Pediatrics published a study linking Guillain-Barré syndrome, an autoimmune disease that causes nerve damage, muscle weakness and paralysis, to the vaccine.(42) The same year the Journal of Korean Medical Science documented acute myelitis(43) and the link with "mental nerve neuropathy" also emerged.(44)
Data then appeared in JAMA on 46 people - mostly women - who had lost their hair after hepatitis B vaccination.(45)
In 1998, both lupus erythematosus and thrombocytopenia were documented in vaccinated subjects(46) and in 1999 more alopecia in the American Journal of Gastroenterology.(47) The same year Autoimmunity documented a demyelinating polyneuropathy, while Neurology published data linking multiple sclerosis and encephalitis to the vaccine.(48-49) Also in 1999, La Nouvelle Presse Médicale wrote about post-vaccination cervical myelitis(50) and in 2000 multiple sclerosis was discussed in Neurology.(51) Also in 2000, the Journal of the Medical Association of Thailand wrote about Guillain-Barré syndrome after recombinant DNA hepatitis B vaccine(52) and in 2001 the Clinical Infectious Diseases documented leukoencephalitis.(53) In 2004, Neurology published a study showing the association between the vaccine and a statistically significant risk of multiple sclerosis;(54) in 2006 the Chinese Medical Journal also documented multiple sclerosis.(55) In 2008, Neurology released two studies showing a statistically significant correlation between hepatitis B vaccination in children and the development of pediatric multiple sclerosis (central nervous system demyelination) more than three years later.(56-57)
Sensory impairment
Numerous medical and scientific publications have documented vision and hearing impairments after hepatitis B vaccination. For example, in 1987 The Lancet published a paper on uveitis – inflammation of the inner lining of the eye that often leads to blindness – after the vaccine.(58) In 1993, again in The Lancet, further data appeared documenting vision loss and eosinophilia - an allergic blood disease - again after vaccination.(59) In 1994 Optometry and Vision Science documented post-vaccination optic neuritis(50) and in 1995 epitheliopathy - a rare eye disorder that causes worsening vision - was discussed in the Archives of Ophthalmology.(61) In 1996, The Lancet published a report documenting “central retinal vein occlusion” after the vaccine,(62) while in the American Journal of Ophthalmology bilateral white spot syndrome was mentioned - which causes loss of vision in both eyes.(63) Also in 1996, La Nouvelle Presse Médicale documented neuropapillitis - inflammation and deterioration of the optic nerve - after the vaccine(64) and another French journal, Annales d'Otolaryngologie et de Chirurgie Cervico-Faciale, mentioned hearing loss.(65) In 1997, La Nouvelle Presse Médicale then published two different studies documenting serious cases of central retinal vein occlusion after vaccination.(66-67) The same year Nephrology Dialysis Transplantation confirmed the onset of optic neuritis after the vaccine(68) and International Ophthalmology certified “ophthalmic complications” in vaccinated subjects.(69) Also in 1997, the Annals of the New York Academy of Sciences and the international journal Auris, Nasus, Larynx noted post-vaccination hearing loss,(70-71) while in 1998 the Journal of French Ophthalmology published data on epitheliopathy.(72) In 1999, the BMJ confirmed optic neuritis after hepatitis B vaccination(73) and Acta Ophthalmologica Scandinavica papilledema - swelling of the optic disc.(74) In 2001, a German magazine, Klinische Monatsblätter Für Augenheilkunde, also confirmed post-vaccination optic neuritis.(75)
Blood diseases
In 1990, soon after the mass market introduction of the hepatitis B vaccine, the BMJ documented vasculitis, an inflammation of the blood vessels, after administration of the drug.(76) In 1993, the English magazine Thorax provided confirmation(77) and The Lancet published a study on eosinophilia, an allergic blood disease, again after vaccination.(78) In 1994 and 1995 The Lancet also documented thrombocytopenia - a serious disease that causes excessive bleeding, bruising and clotting problems.(79-80) In 1998, the onset of thrombocytopenia was confirmed in numerous recently vaccinated patients(81) also from the Scandinavian Journal of Infectious Diseases confirmed and Archives of Disease in Children published data confirming this disease as an adverse event of the vaccine.(82) In 1999, the European Journal of Pediatrics once again confirmed thrombocytopenia after both hepatitis B and MMR vaccines(83) and the same year the Journal of Rheumatology published two important studies of which the first had demonstrated the correlation between vaccine and vasculitis(84) and the second erythermalgia, vascular spasms in the hands and feet that cause pain and burning.(85) In 2000, Clinical and Experimental Rheumatology studied cases of polyarthritis nodosa(86) - a rare, systemic, necrotizing (cell-damaging) type of vasculitis - and the British Journal of Haematology documented severe pancytopenia - a dangerous reduction in red blood cells.(87) In 2001, the Journal of Rheumatology published additional data confirming the possibility of vasculitis after recombinant hepatitis B vaccine(88) and the Italian journal Haematologica confirmed thrombocytopenia as an adverse event.(89)
Skin diseases
In 1989, the New England Journal of Medicine documented erythema nodosum — painful inflammation of the skin with soft bumps — after hepatitis B vaccination.(90) In 1993, the Journal of Rheumatology reported cases of both erythema nodosum and Takayasu arthritis – a rare form of vasculitis.(91) The same year the Swedish journal Acta Dermato-Venereologica wrote about lichen ruber planus after vaccination(92) - an itchy rash on the skin characterized by thick, hard lesions close together that resemble algae or fungi growing on rocks. In 1994, the Archives of Dermatology also documented lichen planus after vaccination(93) and Pediatric Dermatology demonstrated a link to erythema multiforme.(94) In 1997, the Australasian Journal of Dermatology confirmed the “lichenoid reaction” (lichen planus) after the vaccine(95) and the Journal of the American Academy of Dermatology wrote about anethodermia(96) - localized wrinkling, loss of elasticity and atrophy of the skin - after vaccination. In 1998, the British Journal of Dermatology published two studies documenting post-vaccination skin diseases: one was on lichen planus(97) and the other on urticaria and angioedema,(98) allergic-based pathologies characterized by burning, stinging and painful swelling. In 1999, lichen planus was also mentioned in the International Journal of Dermatology(99) and in 2000, data confirming post-vaccination erythema multiforme were published in Clinical and Experimental Dermatology.(100) In the same year, the Nepal Journal of Dermatology again wrote about lichen planus after hepatitis B vaccination(101) in 2001 the mention appeared in the Journal of the American Academy of Dermatology(102) while Pediatric Dermatology spoke of lichenoid eruption.(103)
Diabetes liver and kidney diseases
In 1994 The Lancet documented liver dysfunction after hepatitis B vaccination(104) and in 1995 Clinical Nephrology published a study on nephrotic syndrome - kidney damage - again after vaccination.(105) In 1996 the New Zealand Medical Journal published two documents that correlated antihepatitis B to epidemics of insulin-dependent diabetes mellitus (IDDM). The authors found that in the three years following a newly introduced and very extensive mass vaccination campaign, there was a 60% increase in IDDM cases.(106-107) In 1997, Intensive Care Medicine wrote about liver inflammation and acute respiratory disease after vaccination (108). In 2000, Pediatric Nephrology confirmed the possibility of suffering from nephrotic syndrome after receiving the vaccine.(109) Other publications also documented adverse reactions to this vaccine.(110-111-112-113-114-115-116-117-118-119)
France has eliminated antihepatitis B from the pediatric vaccination calendar
In July 1998, approximately 15.000 French citizens belonging to fifteen associations filed a lawsuit against the French government alleging that it had misled the public about the risks and benefits associated with the hepatitis B vaccine. Hundreds - perhaps thousands - of people had suffered autoimmune diseases and neurological disorders, including multiple sclerosis, after vaccination.(120) As a result, in October 1998 France became the first country to abolish the requirement for this vaccine to be admitted to school.(121)
The hepatitis B AIDS vaccine
In 1978 the New York Blood Center in Manhattan, New York, injected homosexual men with an experimental vaccine against hepatitis B, produced by Merck, for the preparation of which chimpanzees were used. Shortly thereafter, male homosexuals in San Francisco, Los Angeles, Denver, Chicago, and St. Louis also received 3 doses of the drug over a three-month period.
In 1980, 20% of gay men who volunteered for the Manhattan experiment tested positive for HIV - the highest incidence in the world, including Africa. In 1981, the AIDS epidemic became official. Although there is no evidence that the experimental hepatitis B vaccine in those homosexual volunteers caused AIDS, there is no doubt that the disease peaked soon after the shots.(122)
How effective is the hepatitis B vaccine?
The effectiveness of the hepatitis B vaccine was defined by injecting the drug into subjects on whom the specific antibodies produced in the blood were then measured. These antibodies must meet or exceed certain levels established by experts and which are presumed to provide protection. Scientists call it "seroprotection." According to this definition, the vaccine is considered "highly immunogenic" when antibody levels are measurable in the short period after the last dose of a three-booster cycle.(123) Yet, according to the manufacturing industries, the duration of the protective effect in healthy vaccinated people is not known. Follow-up studies as early as five to nine years later show that about half of all vaccinated subjects no longer have protective levels of antibodies.(124-125)
For example, a study published in the New England Journal of Medicine found that after five years, antibody levels (which are presumed to be linked to immunity) had dropped dramatically or were no longer detectable in 42% of those vaccinated. Furthermore, 34 of the 773 subjects (4,4%) were infected with the virus.(126-127) In another study, fewer than 40% of those vaccinated had protective antibodies after five years.(128)
Similar research showed that 48% of vaccinated subjects had inadequate antibody levels after just four years.(129) According to the WHO, up to "60% of adults will lose all measurable antibodies induced by the hepatitis B vaccine within six to ten years."(130) and the medical literature is full of data confirming the failure of vaccination.(131-132)
References
- Jacobson IM et al., "Lack of effect of hepatitis B vaccine on T-cell phenotypes", NEJM, 1984, 311 (16), pp. 1030-1032.
- Institute of Medicine, Adverse Events Associated with Childhood Vaccines: Evidence Bearing on Causality, National Academy Press, Washington, DC, 1994.
- Merck & Co., Inc. Recombivax HB Hepatitis B Vaccine (Recombinant), Package Insert as of June 2005.
- Ibid.
- Ibid.
- Tan LJ, “The hepatitis B vaccine,” American Medical Association, AMA helping doctors help patients, www.arna-assn.org, 9 December 2004.
- Vaccine Adverse Event Reporting System VAERS. Rockville, MD.
- Rogers ton SJ et al . , "Hepatitis B vaccine associated with erythema nodosum and polyarthritis", BMJ, 1990, 301, p. 345.
- Hachulla E. et al., "Reactive arthritis after hepatitis B vaccination Journal of Rheumatology”, 1990, 17, pp. 1250-1251.
- Vautier G. et al., "Acute sero-positive rheumatoid arthritis occurring after hepatitis vaccination", Br. J. Rheumatol., October 1994, 33 (10), p. 991.
- Hassan W. et al ., "Reiter's syndrome and reactive arthritis in healthcare workers after vaccination", BMJ, 9 July 1994, 309 (6967), p. 94.
- Birley HD et al., "Hepatitis B immunization and reactive arthritis", BMJ, December 1994, 309 (6967), p. 1514.
- Gross et al., "Arthritis after hepatitis B vaccination. Report of three cases," Scand. J. Rheumatol., 1995, 24 (1), pp. 50-52.
- Biasi D. et al., "Rheumatological manifestations following hepatitis B vaccination. Report of three cases", Scand. J. Rheumatol., 1995, 24, pp. 50-52.
- Aherne P. et al., "Psoriatic arthropathy", Irish Medical Journal, March-April 1995, 88 (2), p. 72.
- Harrison BJ et al., "Patients who develop inflammatory polyarthritis (PH) after immunization are clinically indistinguishable from other patients with PH," Br. J. Rheum., March 1997, 36 (3), pp. 366-369.
- Bracci M. et al., "Polyarthritis associated with hepatitis B vaccination", Br. J. Rheumatol., February 1997, 36 (2), pp. 300-301.
- Pope JE et al., "The development of rheumatoid arthritis after recombinant hepatitis B vaccination", J. Rheum., September 1998, 25 (9), pp. 1687-1693.
- Grasland A et al., "Adult-onset Still's disease after Hepatitis A and B vaccination?",Rev. Internal Med., February 1998, 19 (2), pp. 134-136.
- Maillefert JF et al., "Rheumatic disorders developed after hepatitis B vaccination", Rheumatol. (Oxford), October 1999, 38 (10), pp. 978-983.
- Toussirot E. et al., "Sjogren's syndrome occurring after hepatitis B vaccination", Arthritis Rheuma, September 2000, 43 (9), pp. 2139-2140.
- Ribera ER et al., "Polyneuropathy associated with administration of hepatitis B vaccine", N. Engl. J. Med., 8 September 1983, 309 (10), pp. 614-615.
- Shaw FE Jr. et al., "Postmarketing surveillance for neurologic adverse events reported after hepatitis B vaccination. Experience of the first three years'', Am. J. Epidemiol., February 1988, 27 (s), pp. 337-352 .
- Biron P. et al., "Myasthenia gravis after general anesthesia and hepatitis B vaccine", Arch. Intern. Med., December 1988, 148 (12), p. 2685.
- Herroelen L. et al. , "Central nervous system demyelination after immunization with recombinant hepatitis B vaccine", The Lancet, 9 November 1991, 338 (8776), pp. 1174-1175.
- Tudela P. et al., "Systemic lupus erythematosus and vaccination against hepatitis B", Nephron, 1992, 62 (2), p. 236.
- Martinez E. et al ., "Evan's syndrome triggered by recombinant hepatitis B vaccine'', Clin. Infect. Dis., 1992, 15, p. 1051.
- Ganry 0. et al., "Peripheral facial paralysis following vaccination against hepatitis B. Apropos of a case", Therapie, 1992, 47, pp. 437-438.
- Waisbren BA, "Other side of the coin (letter)", Inf Dis. News, 1992, 5, p. 2.
- Trevisani F. et al., "Transverse myelitis following hepatitis B vaccination",]. of Hepatology, September 1993, 19 (2), pp. 317-318.
- Mahassin F. et al. , "Acute myelitis after vaccination against hepatitis B", Presse Med., December 1993, 22 (40), pp. 1997-1998.
- Nadler JP, "Multiple sclerosis and hepatitis B vaccination", Clin. Infect. Dis., November 1993, 17 (5), pp. 928-929.
- Mamoux V. et al., "Lupus erthymatosus disseminatus and vaccination against hepatitis B virus", Arch. Pediatr., 1994, 1, pp. 307-309.
- Deisenhammer F. et al., "Acute cerebellar ataxia after immunization with recombinant hepatitis B vaccine", Acta Neurol. Scand., June 1994, 89 (6), pp. 462-463.
- Kaplanski G. et al., "Central nervous system demyelination after vaccination against hepatitis B and HLA haplotype", J. Neurol. Neurosurg. Psychiatry, June 1995, 58 (6), pp. 758-759.
- Tartaglino LM et al., "MR imaging in a case of post vaccination myelitis", Am. J. Neuroradiol., 1995, 16 (3), pp. 581-582.
- Guiserix J., "systemic lupus erythematosus following hepatitis B vaccine", Nephron, 1996, 74 (2), p. 441.
- Grezard P. et al., "Cutaneous lupus erythematosus and buccal aphthosis after hepatitis B vaccination in a 6-year old child", Ann. Dermatol. Venereal., 1996, 123 (10), pp. 657-659.
- Manna R. et al., "Leukoencephalitis after recombinant hepatitis B vaccine", J. of Hepatology, June 1996, 24 (6), pp. 764-765.
- Mathieu E. et al ., "cryoglobulinemia after hepatitis B vaccination", New England]. Med., August 1996, 335 (5), p.335.
- Cohen AD. et al., "Vaccine-induced autoimmunity", J. Autoimmunity, December 1996, 9 (6), pp. 699-703.
- Kakar A. et al., "Guillain Barre syndrome associated with hepatitis B vaccination", Ind. J. Ped., September-October 1997, 64 (5), 710-712.
- Song HK et al., "Acute Myelitis after hepatitis B vaccination", J. Korean Med. Sci., June 1997, 12 (3), pp. 249-251.
- Maillefert JF et al., "Mental nerve neuropathy as a result of hepatitis B vaccination," Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology, June 1997, 83 (6), pp. 663-664.
- Wise RP et al., "Hair loss after routine immunizations," JAMA, October 8, 1997, 278 (14), pp. 1176-1178.
- Finielz P. et al, "Systemic lupus erythematosus and thrombocytopenic purpura in two members of the same family following hepatitis B vaccine", Nephrol. Dial. Transplant., 1998, 13 (9), pp. 2420-2421.
- Flemrner M. et al., "The bald truth", Am. J. Gastroenterol., April 1999, 94 (4), p. 1104.
- Creange A et al., "Lumbosacral acute demyelinating polyneuropathy following hepatitis B vaccination", Autoimmunity, 1999, 30, pp. 143-146.
- Tourbah A. et al., "Encephalitis after hepatitis B vaccination: recurrent disseminated encephalitis or MS?", Neurology, 22 July 1999, 53 (2), pp. 396-401.
- Renard JL et al., "Acute transverse cervical myelitis following hepatitis B vaccination. Evolution of anti-HBs antibodies", Presse Med., 3-10 July 1999, 28 (24), pp. 1290-1292.
- Gran B. et al., "Martin R. Development of multiple sclerosis after hepatitis B vaccination", Neurol., 2000, 54 (suppl 3), A164.
- Sinsawaiwong S. et al., "Guillain-Barre syndrome following recombinant hepatitis B vaccine and literature review", J. Med. Assoc. Thai., September 2000, 83 (9), pp. 1124-1126.
- Konstantinou D. et al ., ''Two episodes of leukoencephalitis associated with recombinant hepatitis B vaccination in a single patient', Clin. Inf. Dis., 15 November 2001, 33, pp. 1772-1773.
- Heman MA et al., "Recombinant hepatitis B vaccine and the risk of multiple sclerosis: a prospective study", Neurology, 2004, 63, pp. 838-842.
- Terney D. et al., "Multiple sclerosis after hepatitis B vaccination in a 16-year-old patient", Chinese Medical Journal, 2006, 119 (1), pp. 77-79.
- Yann M. et al., “Hepatitis B vaccine and the risk of CNS inflammatory demyelination in childhood,” Neurology, October 8, 2008 [published online].
- Ness JM et al “Hepatitis vaccines and pediatric multiple sclerosis. Does timing or type matter?”, Neurology, 17 December 2008 (published online).
- Fried Met al., "Uveitis after hepatitis B vaccination", The Lancet, 12 September 1987, pp. 631-632.
- Brezin A. et al., "Visual loss and eosinophilia after recombinant hepatitis B vaccine", The Lancet, 28 August 1993, 342 (8870), pp. 563-564.
- Achiron LR et al., "Postinfectious hepatitis B optic neuritis", Optom. View Sci., 1994, 71, pp. 53-56.
- Brezin AP et al., "Acute posterior multifocal placoid pigment epitheliopathy after hepatitis B vaccine", Arch. Ophthalmol., March 1995, 113 (3), pp. 297-300.
- Devin F. et al., "Occlusion of central retinal vein after hepatitis B vaccination", The Lancet, June 1996, 347 (9015), p. 1626.
- Baglivo E. et al., "Multiple evanescent white dot syndrome after hepatitis B vaccine", Am. J. Ophthalmol., September 1996, 122 (3), pp. 431-432.
- Berkman N. et al., "Bilateral neuro-papillitis after hepatitis B vaccination", Presse Med., 28 September 1996, 25 (28), p. 1301 (French).
- Bonfils P. et al., "Fluctuant perception hearing loss after hepatitis B vaccine", Ann. Otolaryngol. Chir. Cervicofac., 1996, 113 (6), pp. 359-361 (French).
- Granel B. et al., "Occlusion of the central retinal vein after vaccination against viral hepatitis B with recombinant vaccines. 4 cases", Presse Med., 1 February 1997, 26 (2), pp. 62-65 [French].
- Berkman N., "A case of segmental unilateral occlusion of the central retinal vein following hepatitis B vaccination", Presse Med., 26 April 1997, 26 (14), p. 670 [French].
- Albitar S. et al., "Bilateral retrobulbar optic neuritis with hepatitis B vaccination", Nephrol. Dial. Transplant, October 1997, 12 (10), pp. 2169-2170.
- Arya SC, "Ophthalmic complications of vaccines against hepatitis B virus", Int. Ophth., 1997, 21 (3), pp. 177-178.
- Orlando MP et al., "Sudden hearing loss consequent to hepatitis B vaccination: a case report", Annals of the New York Academy of Sciences, 29 December 1997, 830, pp.319-321.
- Biacabe B. et al., ”A case report of fluctuant sensorineural hearing loss after hepatitis B vaccination”, Auris, Nasus, Larynx, October 1997, 24, (4), pp. 357-360.
- Bourges JL et al., “Multifocal placoid epitheliopathy and anti-hepatitis B vaccination", J. Fr. Ophtalmol., November 1998, 21(9), pp. 696-700 [French].
- Stewart O. et al ., “Simultaneous administration of hepatitis B and polio vaccines associated with bilateral optic neuritis", Br. J. Ophthalmol., October 1999, 83 (10), pp.1200-1201.
- Fledelius HC, “Unilateral papilledema after hepatitis B vaccination in a migraine patient. A case report including forensic aspects", Acta Ophthalmol. Scand., December 1999, 77 (6), pp. 722-724.
- Voigt u. et al ., “Neuritis of the optic nerve after vaccinations against hepatitis A hepatitis B and yellow fever”, Klin. Monatsbl. Augenheilkd., October 2001, 218 (10), pp. 688-690 (German).
- Cockwell P. et al., "Vasculitis related to hepatitis B vaccine", BMJ, 1 December 1990, 301 (6763) p.1281.
- Allen MB et al., "Pulmonary and cutaneous vasculitis following hep B vaccination", Thorax, May 1993, 48 (5), pp. 580-581.
- Nagafuchi S. et al., "Eosinophilia after intradermal hepatitis B vaccination", The Lancet, 1993, 342, p. 998.
- Poullin P. et al., "Thrombocytopenic purpura after recombinant hepatitis B vaccine", The Lancet, November 1994, 344 (8932), p. 1293.
- Meyboom RH et al., "Thrombocytopenia reported in association with hepatitis B and A vaccines", The Lancet June 1995, 345 (8965), p. 1638.
- Neau D. et al., "Immune thrombocytopenic purpura after recombinant hepatitis B vaccine: retrospective study of seven cases", Scan J. Infect Dis., 1998, 30 (2), pp. 115-118.
- Ronchi F. et al., "Thrombocytopenic purpura as adverse reaction to recombinant hepatitis B vaccine", Arch. Dis. Child., March 1998, 78 (3), pp. 273-274.
- Muller A. et al., "Thrombocytopenic purpura: adverse reaction to combined immunization (recombinant hepatitis B and measles-mumps-rubella-vaccine) and after therapy with Co-trimoxazole", Eur.]. Pediatr., December 1999, 158 Suppi. 3, S209-10.
- Le Hello C. et al., “Suspected hepatitis B vaccination related vasculitis", J. of Rheumatology, January 1999, 26 (1), pp. 191-194.
- Rabaud C. et al., "First case of erythermalgia related to hepatitis B vaccination", J. of Rheum., January 1999, 26 (1), pp. 233-234.
- De Keyser F. et al., “Immune-mediated pathology following hepatitis B vaccination” Two cases of polyarteritis nodosa and one case of pityriasis rosea-like drug eruption”, Clin. Exp. Rheumatol., January-February 2000, 18 (1), pp. 81-85.
- Viallard JF et al., "Severe pancytopenia triggered by recombinant hepatitis B vaccine", Br. J. Haematol., July 2000, 110 (1), pp. 230-233.
- Zaas A. et al., uLarge artery vasculitis following recombinant hepatitis B vaccination. 2 cases", J. Rheumatol., May 2001, 28 (S), pp.1116-11120.
- Conesa V. et al., "Thrombocytopenic Purpura after Recombinant Hepatitis B Vaccine. A rare association", Haematologica, March 2001, 86 (3), E09 [Italian].
- Goolsby PL, “Erythema nodosum after Recombivax HB hepatitis B vaccine,” N. Engl. J. Med., October 1989, 321, 1198-1199.
- Castresana-Isla CJ et al ., "Erythema nodosum and Talkayasu's arteritis after immunization with plasma derived hepatitis B vaccine", J. Rheumatol., August 1993, 20(8), pp. 1417-1418.
- 109. Trevisian G. et al., "Lichen ruber planus following HBV vaccination". Acta Dermato-Venereotogica, February 1993, 73 (1), p. 73.
- Aubin F. et al., “Lichen planus following hepatitis B vaccination,” Archives of Dermatology, October 1994; 130 (10), pp. 1329-1330.
- Di Lernia V. et al., "Bisighini G. Erythema multiforme following hepatitis B vaccine", Ped. Derma., December 1994, 11 (4), pp. 363-364.
- Saywell CA. et al., "Kossard S. Lichenoid reaction to hepatitis B vaccination", Australasian J. Derm., August 1997, 38 (3), pp. 152-154.
- Dauod MS et al., “Anetoderma after hepatitis B immunization in two siblings,”/. Amer. Acad. Dermatol., May 1997, 36 (5 Pt 1), pp. 779-780.
- Ferrando MF et al., "Lichen planus following hepatitis B vaccination” , Br. J. Dermatol., August 1998, 139 (2) p. 350.
- Barbaud A. et al ., "Allergic mechanisms and urticaria/angioedema after hepatitis B immunization", Bt. J. Dermatot, November 1998, 139 (5), pp. 925-926.
- Schupp P. et al., "Lichen planus following hepatitis B vaccination", Inter. J. of Dermat., October 1999, 38 (10), pp. 799-800.
- Loche F. et al., "Erythema multiforme associated with hepatitis B immunization", Cfin. Exp. Dermatol., March 2000, 25 (2), pp. 167-168.
- Agrawal S. et al., "Lichen planus after HBV vaccination in a child: a case report from Nepal", J. Dermatol., September 2000, 27 (9), pp. 618-620.
- Al-Khenaizan S., "Lichen planus occurring after hepatitis B vaccination: a new case", J. Am. Acad. Dermatol., October 2001, 4S (4), pp. 614-615.
- Usman A. et al ., “Lichenoid eruption following hepatitis B vaccination: first North American case report". Pediatr. Dermato J., Mar-Apr. 2001, 18 (2) pp. 123-126.
- Lilic D. et al., “Liver dysfunction and DNA antibodies after hepatitis B vaccination", The Lancet, November 1994, 344 (8932), pp. 1292-1293.
- Macario F. et al., “Nephrotic syndrome after recombinant hepatitis B vaccine", Clin. Nephrol., May 1995, 43 (5), p. 349.
- Classen John Barthelow, "Childhood immunization and diabetes mellitus", New Zealand Med. J., 24 May 1996, p. 195.
- Classen John Barthelow, ''The diabetes epidemic and the hepatitis B vaccine", New Z. Med. J., 24 May 1996, p. 366.
- Ranieri VM et al., "Liver inflammation and acute respiratory distress syndrome in a patient receiving hepatitis B vaccine: a possible relationship?", Intensive Care Medicine, January 1997, 23 (1), pp. 119-121.
- Islek I. et al., “Nephrotic syndrome following hepatitis B vaccination", Pediatr. Nephrol., January 2000, 14, pp. 89-90.
- Snider GB et al., “A possible systemic reaction to hepatitis B vaccine,” JAMA. March 1, 1985, 253 (9), pp. 1260-1261.
- AADRAC, "Australian Adverse Drug Reactions Advisory Committee: Reactions to hepatitis B vaccines", Australian Adverse Drug Reactions Bulletin, August 1990.
- Germanaud J. et al., "A case of severe cytolysis after hepatitis B vaccination", Amer. J. Med., June 1995, 98 (6), pp. 595-S96.
- Fisher BL, Ed., "Hepatitis B vaccine: the untold story", The Vaccine Reaction, National Vaccine Information Center, September 1998.
- Belkin Michael, “Government-mandated thalidomide for babies,” WorldNetDaily, January 25, 1999, worldnetdaily.com
- Howd A., “Ounce of Prevention, Pound of Misery,” Insight Magazine, March 12, 1999,
- Bethell T., “Shots in the Dark". American Spectator. May 1999.
- Wallstin B., “Immune to Reason,” The Houston Press, June 3, 1999, www.houstonpress.com
- Shaw FE, “Uproar over a little known preservative, thimerosal, jostles US hepatitis B vaccine policy”, Hepatitis Control Report, Summer 1999, Vol. 4, no. 2.
- Spalding BJ, “Miracle or murder? The hepatitis B vaccine controversy", Biospace.com, 11 November 1999, www.biospace.com
- Science, 31 July 1998.
- “France suspends use of hepatitis B vaccine”. https://www.bmj.com/content/317/7165/1034.2
- Cantwell A., "The gay experiment that started AIDS in America”, Not Aids, January 13, 2006, now in http://rense.com/general68/gayex.htm
- Merck & Co.t Inc., Recombivax HB Hepatitis B Vaccine (Recombinant), Package Insert as of June 2005; GlaxoSmithKline Biologicals, Engerix-B (Hepatitis B Vaccine (Recombinant), Package Insert as of December 2005.
- Wainwright RB et al., "Duration of immunogenicity and efficacy of hepatitis B vaccine in a Yupik Eskimo population, preliminary results of an 8-year study", in “Viral hepatitis and liver disease”, Hollinger FB et al. (eds.) , Williams & Wilkins, 1990, pp. 762-766.
- Hadler SC et al., “Evaluation of long-term protection by hepatitis B vaccine for 7 to 9 years in homosexual men.” in Viral hepatitis and liver disease, Hollinger FB et al. (eds..), Williams & Wilkinst 1990, pp. 766-768.
- Hadler SC et al., “Long-term immunogenicity and efficacy of hepatitis B vaccine in homosexual men,” NEJM, July 24, 1986, 315, pp. 209-214.
- Stevens CE et al., "Prospects for control of hepatitis B virus infection: implications of childhood vaccination and long-term protection", Pediatrics, 1992, 90, pp. 170-173.
- Street AC et al., "Persistence of antibody in healthcare workers vaccinated against hepatitis B", Infec. Control Hosp. Epidem., 1990, 11, pp. 525-530.
- Pasko MT et al., "Persistence of anti-HBs among health care personnel immunized with hepatitis B vaccine", American Journal of Public Health, 1990, 80, pp. 590-593.
- World Health Organization, ccHepatitis B vaccines: immunogenicity reappraised'', WHO Drug Infor., 1994, 8 (2).
- Ballinger, AB et al., "Severe acute hepatitis B infection after vaccination”, The Lancet, 1994, 344, pp.1292-1293
- Goffin E. et al., "Acute hepatitis B infection after vaccination", The Lancet, 1995, 345, p. 263.
Vaccines: A Guide to Informed Choice by Neil Z. Miller (Author) and Claudia Benatti (Translator)
Terra Nuova Editions, 2018

