Pharmacovigilance: an all-Italian failure

With this article of ours we will try to shed light on the vast world of Pharmacovigilance, summarizing the basic concepts that characterize it and attempting to analyze the official reports of the Italian Medicines Agency (AIFA) in order to make more concrete what is too often completely theoretical.
Pharmacovigilance plays a very important role in the field of medicines, but becomes crucial in relation to vaccines as they are designed to be administered to healthy subjects, often at a very young age. While on the one hand we always invite all our supporters to cultivate doubt, to investigate independently and to always check the sources, including our own, when it comes to pharmacovigilance for vaccines we are too often faced with attitudes that border on gullibility. popular; almost a faith with its own liturgies that uncritically addresses the topic and establishes the goodness of the practice without ifs or buts, with a radical and fideistic rejection of the idea that like all drugs, Even vaccines can harm your health.
We understand the context
To begin to understand what we are talking about, we must define the meaning of two very important terms: Pharmacovigilance e Adverse reaction. We will help ourselves with the official definitions given by the Italian Medicines Agency (AIFA), the European Regulations and Directives,(1-2-3) and by the World Health Organization:
- Pharmacovigilance: “...is the science of activities that refer to the recognition, evaluation, understanding and prevention of adverse effects and any other problems related to drugs.”(4)
- Adverse reaction: “...a harmful and unwanted effect resulting not only from the authorized use of a medicinal product under normal conditions of use, but also from therapeutic errors and uses that do not comply with the authorized indications, including the improper use and abuse of the medicinal.”(5)
The concept of adverse reaction should be extended to also include the lack of therapeutic efficacy, in particular, in order to highlight potential signals of reduced immunogenicity in a subgroup of vaccinated people (classic example non-responders), of declining immunity (often falling into failure vaccine) or replacement of the strain (with Covid19 we saw it very well) and the importance of also observing this decisive aspect is explained very well in the general indications on monitoring the failure of vaccines provided by the "Report of CIOMS/WHO Working Group on Vaccine Pharmacovigilance".(6)
In this regard, AIFA issued a circular in May 2021(7) where he further detailed the problem of reporting lack of efficacy, reiterating the need for reporting to the National Pharmacovigilance Network (RNF) even without a suspected adverse reaction being found in the case of vaccines.
The Italian pharmacovigilance system is based on the AIFA National Pharmacovigilance Network (RNF),(8) which should guarantee the collection, management and analysis of reports of suspected adverse drug and vaccine reactions (ADR Adverse Drug Reaction in English).
The reporting of any suspected adverse reaction must be reported by all healthcare professionals (doctors, pharmacists, nurses, healthcare assistants, etc.), but also by citizens through the AIFA online platform called VigiFarmaco(9) and, following a complicated form to fill in, the report is sent to the Pharmacovigilance Manager who, if valid, automatically inserts it into the National Pharmacovigilance Network (NFR).
We need to make another specification, perhaps one of the most important, and that is the division of Pharmacovigilance into Passive pharmacovigilance e Active pharmacovigilance:
- Passive pharmacovigilance: it is the method that is used most frequently in passive post-marketing surveillance and consists of a solely spontaneous reporting system where citizens and healthcare professionals spontaneously decide to report a suspected adverse reaction. It is a simple, practical tool, has a very low cost and requires very limited organizational resources.
- Active pharmacovigilance: Contrary to passive testing, it seeks to fully ascertain all adverse events, through a continuous pre-organized process. In general, it is more feasible to recover complete data on reports of an individual adverse event through an active surveillance system rather than through a passive surveillance system and this is done by interviewing patients and/or doctors in sentinel sites, in order to guarantee complete data and accurate on adverse events over time. It can also be accomplished through review of medical records. It has a high cost and requires huge organizational energies.
Now that you have a general overview of passive Pharmacovigilance through the VigiFarmaco portal, we invite you to check it out for yourself, asking friends and relatives, those who know this specific portal or those who even just know about the possibility and usefulness of reporting any suspected adverse reaction. We are more than certain that no one will answer that they know about it and this is the first major obstacle to Pharmacovigilance.
The issue of doctors "obliged" to report
We now come to the age-old problem of healthcare workers who they should report any suspected adverse reaction. The Decree of the Ministry of Health of 30 April 2015(10) reiterated the obligation of healthcare personnel to promptly report suspected adverse reactions and defined time limits within which they are required to report. In particular, suspected adverse reactions must be reported within 2 days of the doctor or healthcare professional becoming aware of them and the obligation drops to 36 hours in the case of adverse reactions from medicinal products of biological origin, including vaccines.
These peremptory indications have a very small problem: they completely lack the sanctioning aspect for failure to report. The legislator, in transposing the European Directives from which the Decree arose, did not want to contemplate a sanction in the event of non-compliance and this effectively makes this obligation an exercise of a purely philosophical, almost mythological nature given its rarity.
Therefore, the doctor is not obliged to report suspected adverse reactions and the citizen is not aware of the possibility of reporting spontaneously... what could possibly go wrong?
We would like to inform you that at an international level several studies have defined the problem of under-reporting of adverse reactions, estimating the detection of ADRs by passive pharmacovigilance, compared to real adverse reactions, between 1 and 10 per cent. hundred. This means that only a tenth or even a hundredth of actual vaccination reactions are reported.(11-12)
An active Pharmacovigilance study on the MPRV vaccine (Measles-Mumps-Rubella-Varicella) carried out by the Puglia Region,(13) had found that the serious adverse reactions collected with active Pharmacovigilance exceeded the reports received spontaneously with passive Pharmacovigilance by 339 times and, again in this study, it was demonstrated that the problem of under-reporting weighs especially on serious adverse reactions.
Another study from 2018(14) demonstrated instead that the problem of under-reporting also exists on drugs subjected to additional monitoring (medicines subject to close and specific monitoring by regulatory agencies) and, even in this case, it was noted that the greatest problem falls precisely on the serious adverse reactions, including deaths.
Let's not raise the question of additional monitoring now because it would require too broad a parenthesis, but just think that once a drug or vaccine passes phase III testing, the next step is called post-marketing, or phase IV. From this phase onwards the product is marketed and for 4 years it must carry a small black triangle on the information leaflet which determines that it is a medicine undergoing additional monitoring and all adverse reactions must be reported. This never happens.
Let's just talk about vaccines now
From here on we will only take into consideration the vaccines segment and to do so we need to add a new definition: Vaccinovigilance.
Vaccinovigilance: means the set of pharmacovigilance activities relating to the "collection, evaluation, analysis and communication of adverse events following immunization (AEFI - Adverse Event Following Immunization)".(15-16)
Vaccinovigilance activity in Italy is carried out by the Vaccinovigilance Working Group established by AIFA with the Resolution of 30 July 2014.(18-19) This group is made up of representatives of the Ministry of Health, the Istituto Superiore di Sanità and the Regional Pharmacovigilance and Prevention Centers and has the objective of managing and investigating any signals coming from reports of suspected adverse reactions to vaccines included in the National Network of Pharmacovigilance, the in-depth analysis of relevant topics in the regulatory or scientific field, the production and dissemination of documents useful for the post-marketing management of vaccines (such as the Guide to the evaluation of adverse reactions observable after vaccination).
Let's get to reality
Summing up to the maximum what we have learned so far, we can say that Vaccinovigilance consists in the collection and analysis of suspected adverse reactions to vaccines, endemically underestimated and reported in a completely spontaneous way by citizens totally unaware of the possibility and the process to be followed and by doctors who do not have the will and perhaps not even the ability to recognize an adverse reaction. Does our vision seem too critical to you?
In order to get into the reality of the facts and try to understand the real numbers of the phenomenon, we can use the AIFA reports on the postmarketing surveillance of vaccines in Italy in 2020 and 2021. We will use these reports on non-Covid19 vaccines because our interest is to analyze the "normal" situation regarding Pharmacovigilance, certain of the fact that the Covid-19 "Pandemic" has led to a further worsening of the situation. Think about this: if what we are about to show you presents serious statistical problems on the Vaccinovigilance of those vaccines administered annually to your children, or to yourself, in a routine way, in the case of Covid19 vaccines could the situation ever be better? In our opinion it can only be drastically worse...
Let's get to AIFA's vaccine reports and start reeling off some data so we can start thinking about it:
Table 1: comparison of the 2020 Vaccine Report vs. the 2021 Vaccine Report
Attention: if you look at the table above smartphone, scroll to the right to read all the data
| Vaccine Report 2020 | Vaccine Report 2021 |
| 20 million doses administered | 20,5 million doses administered |
| 5.352 reports entered | 18.060 reports entered |
| 3.681 cases relating to 2020 | 15.681 cases relating to 2021 |
| 17,9 reports per 100.000 doses administered | 78 reports per 100.000 doses administered |
This table helps us show you the first anomaly between the two years, we mention: “...2021 is characterized by a substantial increase in reports (approximately 10 times more than the previous year)” due to the fact that more active Pharmacovigilance projects have been activated”.
To understand better: only 2.045 spontaneous reports (passive pharmacovigilance) were collected in 2021, 11,3% of the total, while 2.376 were collected in 2020. The data, as we will see shortly, are therefore in line with the spontaneous reports collected in previous years.
Table 2: annual comparison of suspected adverse reactions collected in AIFA vaccine reports
Attention: if you look at the table above smartphone, scroll to the right to read all the data
| 201823 | 201924 | 2020 | 2021 |
| 3.768 spontaneous reports | 3.834 spontaneous reports | 2.376 spontaneous reports | 2.045 spontaneous reports |
To understand the state of the art of Vaccinovigilance in Italy, the data collected by active Pharmacovigilance should therefore be divided and analyzed separately from the passive one. Instead, our beloved AIFA is keen to keep them together, thus making us think that the spontaneous detection system is progressively improving.
Shall we play a little game? If 2021 spontaneous reports were collected in 2.045 on 20,5 million doses, the 13.921 active Pharmacovigilance reports of the same year on how many doses were detected? We don't know...
Table 3: Active Pharmacovigilance projects included in AIFA's 2021 Vaccines Report
Attention: if you look at the table above smartphone, scroll to the right to read all the data
| Project name | Number of doses/subjects | Adverse reactions | Serious adverse reactions |
| “Active surveillance of adverse events after anti-meningococcal B vaccination” | 1100 children | 880 | 7 |
| “Surveillance of adverse events after vaccination during pregnancy” | Data unknown(a) | Data unknown(a) | Data unknown(a) |
| “VigifarmacoVax: surveillance of adverse reactions from vaccines” | 47.000 SMS(B) | 13.800 records found | Data unknown(B) |
(a) We can't find any news on the project "Surveillance of adverse events after vaccination during pregnancy", not even the Apulian Health website can help us as the dedicated page() is empty.
(B) The “VigifarmacoVax: surveillance of adverse reactions from vaccines” project instead tells us a little something, if only a little. This involves active surveillance of children subject to vaccination in the first two years of life with the primary objective of increasing parental involvement in vaccine vigilance. We do not know how many subjects were recruited other than the data of 47.000 SMS received in response, but we know that there were approximately 13.800 reports of adverse reactions.
If we add the reactions collected by the two active Pharmacovigilance projects for which we have some data, we can know that they collected a total of approximately 14.680 adverse reactions (13.800+880).
Another fact we would like you to focus on: the active Pharmacovigilance of the Puglia Region on the anti-meningococcal B vaccine (Bexsero), has certified 880 adverse events on 1100 children relating to 2021, of which 7 can be classified as serious. If in 2021 they had only activated active Pharmacovigilance, thus calculating the rate on 100.000 doses administered, would they have detected almost 73.000 adverse reactions for this vaccine alone and almost 640 serious ones?
Let's move on, it's better...
Let's go back to the 2020 Report which, having fewer active Pharmacovigilance projects, can help us to better understand the regional situation, that is, to understand if a doctor from Soave, in the province of Verona, has the same sensitivity and attention to Pharmacovigilance compared to a doctor from Lampedusa or Naples or Rome.
Before presenting the next table we must point out that since 2017 our dear and beloved friends at AIFA have stopped indicating the number of reports for each region and this makes it less intuitive to understand that something is not right. Now let's see it in the original and then we'll work on it a little:
Table 4: regional reporting rates per 100.000 inhabitants, extracted from the 2020 Vaccine Report
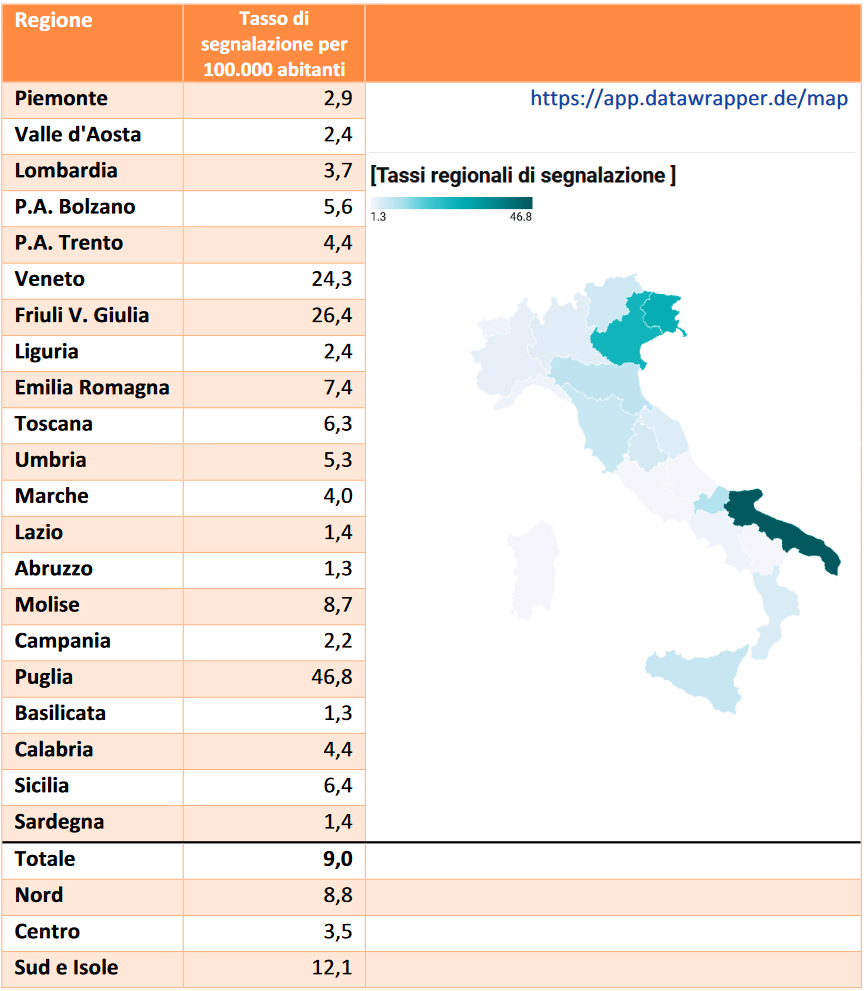
As you can see, the table only shows the reporting rate per 100.000 inhabitants and, as we were saying, the total number of reports of suspected adverse reactions collected for each reason is omitted; Nonetheless, we have enough elements to be able to extrapolate this data ourselves and also carry out the necessary checks. Now we can start rewriting the 2020 Vaccine Report table with clear data:
Table 5: Active Pharmacovigilance projects included in AIFA's 2021 Vaccines Report
Attention: if you look at the table above smartphone, scroll to the right to read all the data
| Region | Inhabitants by region(25) | Reporting rate | Estimated number of reports |
| Piemonte | 4.273.210 | 2,9 | 124 estimated reports |
| Aosta Valley | 123.895 | 2,4 | 3 estimated reports |
| Lombardia | 9.966.992 | 3,7 | 369 estimated reports |
| PA Bolzano | 533.715 | 5,6 | 30 estimated reports |
| PA Trento | 544.745 | 4,4 | 24 estimated reports |
| Veneto | 4.852.453 | 24,3 | 1179 estimated reports |
| Friuli V. Giulia | 1.198.753 | 26,4 | 316 estimated reports |
| Liguria | 1.509.805 | 2,4 | 36 estimated reports |
| Emilia Romagna | 4.445.549 | 7,4 | 329 estimated reports |
| Toscana | 3.668.333 | 6,3 | 231 estimated reports |
| Umbria | 865.013 | 5,3 | 46 estimated reports |
| Marche | 1.501.406 | 4,0 | 60 estimated reports |
| Lazio | 5.720.796 | 1,4 | 80 estimated reports |
| Abruzzo | 1.285.256 | 1,3 | 17 estimated reports |
| Molise | 296.547 | 8,7 | 26 estimated reports |
| Campania | 5.679.759 | 2,2 | 125 estimated reports |
| Puglia | 3.926.931 | 46,8 | 1837 estimated reports |
| Basilicata | 547.579 | 1,3 | 7 estimated reports |
| Calabria | 1.877.728 | 4,4 | 82 estimated reports |
| Sicilia | 4.840.876 | 6,4 | 309 estimated reports |
| Sardinia | 1.598.225 | 1,4 | 22 estimated reports |
| Total estimated reports 5252 |
The first verification of the correctness of the data extrapolated by us is provided by the 2020 Vaccine Report itself:
Compared to our result, 5.252 reports of suspected adverse reactions, the official data contains only 100 more reports, probably due to the fact that the reporting rates on which we have done the calculations have only one decimal place after the decimal point such as to round off our estimates.
However, it is not enough for us and we want to do a further check and since our association has been operating in Veneto since 1993 we know well how to do it. The Veneto Region has had its own Vaccinovigilance system called "Green Channel" since the end of the 90s and annually produces reports on its activities.(26) Having the precise regional data, we compared our result to the "XXIV Report on the activities carried out by Canale Verde for the year 2020"(27) so as to be able to verify that, in the Veneto Region alone, approximately 1200 reports of non-Covid-19 vaccines have been reported (1.653 minus 27% of reports for Covid19 vaccines). We can therefore say that this verification is also positive.
Italian statistical madness
Together we understood the vast world of pharmacovigilance and understood its dynamics and rules; then we focused on vaccine vigilance, on the fake reporting obligation by healthcare workers and, finally, we managed to extrapolate data on which to try to make a real analysis of the situation but now, what conclusions can we draw? We could end this article by saying that, in our very modest opinion, it is a total fool to consider any vaccine "safe" when operating this vaccinevigilance! Since the Veneto Region - whose abnormal underestimation of adverse reactions we have been criticizing for years - collects over 50% of the suspected adverse reactions in all of Italy, then this vaccinevigilance simply does not work! If in most of Italy doctors do not know or do not want to recognize an adverse reaction, the inevitable logical consequence is that when they tell you that vaccines are safe, they are not saying it based on data, but solely as a function of will. of the medical profession not to recognize or report any suspected adverse reaction as required by law! This is neither science nor medicine, but empty scientism!
Side note - To avoid it being said that we have selected a particular year for the sole purpose of pleading a thesis, we also report the 2017 Vaccine Report,(28) again drawn up by AIFA, it shows a situation similar to that analyzed in 2020, with the difference that the data was made quite clear.
Table 6: distribution of reports entered in 2017, extracted from Canale Verde year 2017
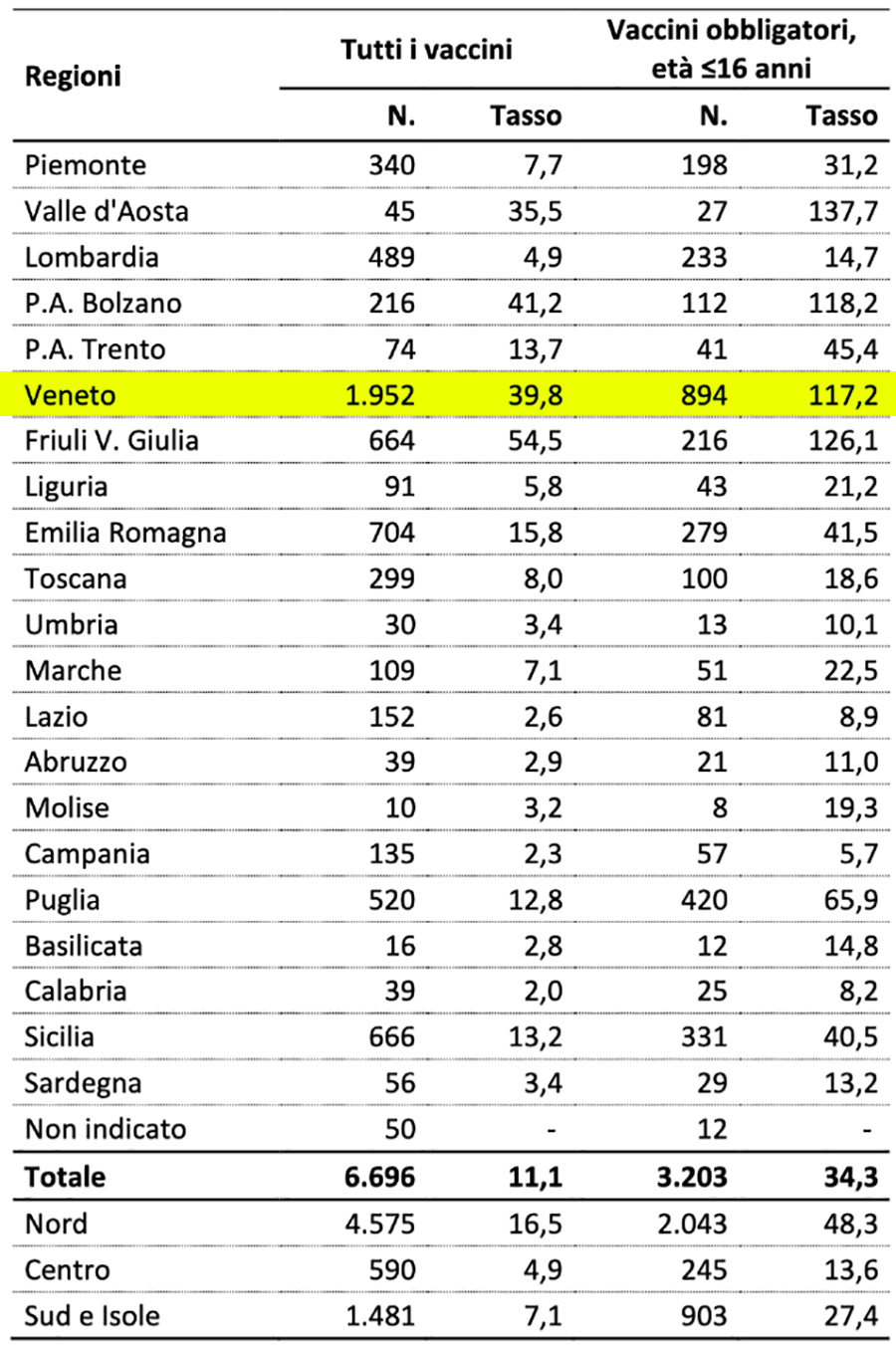
As you can see, in 2017 the Veneto Region alone had collected 1.952 reports of suspected adverse reactions, i.e. 46% of those collected in the rest of the Italian regions and this indicates that the serious underestimation of reports of adverse reactions by the healthcare sector continues for years and shows no signs of decreasing, on the contrary. With the progressive increase in the vaccination offer, there is a slow and progressive decrease in the number of reports collected - identical vaccines, more administrations, fewer reactions - and this trend shows no signs of abating. We remind you that we are talking about non-Covid19 vaccines... for those we will have to worry.
References (click to open)
- DIRECTIVE 2012/26/EU
- EU Regulation 1235/2010
- Directive 2010/84 / EU
- https://www.aifa.gov.it/farmacovigilanza1
- https://www.aifa.gov.it/sites/default/files/Definizioni.pdf
- https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-vi-management-reporting-adverse-reactions_en-0.pdf
- https://www.aifa.gov.it/documents/20142/1135360/Gestione_segnalazioni_Inefficacia_RNF_Maggio_2021.pdf
- https://www.aifa.gov.it/rete-nazionale-di-farmacovigilanza
- https://www.vigifarmaco.it
- https://www.gazzettaufficiale.it/eli/id/2015/06/23/15A04666/sg
- Guidelines for the Ethical Conduct of Studies to Evaluate Drugs in Pediatric Populations - http://pediatrics.aappublications.org/content/pediatrics/60/1/91.full.pdf
- https://link.springer.com/article/10.1007/s40264-023-01302-7
- https://www.corvelva.it/approfondimenti/farmacovigilanza/studio-sul-vaccino-mpvr-in-puglia-eventi-avversi-per-4-bimbi-su-100.html
- https://www.giornaledicardiologia.it/archivio/2852/articoli/28779/
- https://www.who.int/groups/global-advisory-committee-on-vaccine-safety/topics/aefi/serious-aefi
- Global manual on surveillance of adverse events following immunization - https://www.who.int/publications/i/item/10665206144
- https://www.aifa.gov.it/en/-/aifa-la-vaccinovigilanza-in-italia-ruolo-ed-obiettivi-
- https://www.aifa.gov.it/sites/default/files/determina_vaccinovigilanza.pdf
- https://www.aifa.gov.it/-/istituito-gruppo-di-lavoro-per-la-vaccinovigilanza-01-08-2014-
- AIFA Vaccines Report 2020
- AIFA Vaccines Report 2021
- AIFA Vaccines Report 2018
- AIFA Vaccines Report 2019
- https://www.sanita.puglia.it/web/pugliasalute/progetto-valore
- https://www.istat.it/storage/ASI/2021/capitoli/C01.pdf
- https://www.aovr.veneto.it/area-scientifica/vaccinazioni/canale-verde
- https://www.aovr.veneto.it/documents/20182/43721/XXIV+RELAZIONE+SULL%E2%80%99ATTIVITA+canale+verde+2020_.pdf
- https://www.aifa.gov.it/sites/default/files/Rapp_Vaccini_2017_0.pdf

