MPVR vaccine study in Puglia: adverse events for 4 out of 100 children

In 2018, after the approval of the infamous Lorenzin Law, the Regional Epidemiological Observatory of the Puglia Region published a report(1) on the surveillance of adverse events to vaccination for the years 2013-2017, which included a Pharmacovigilance study active on the Priorix Tetra,(2) an MPRV (measles-mumps-rubella-chickenpox) vaccine produced by GlaxoSmithKline.
Let us remember that Pharmacovigilance active, unlike the passive one, it tries to intercept all suspected adverse events through an ongoing pre-organized process; takes place by interviewing patients and/or doctors in sentinel sites, in order to guarantee complete and accurate data over time.
If you want to learn more, we talked to you about pharmacovigilance in one of our articles that you can read on our website.(3)
In the study of the Puglia Region for the MPRV vaccine, all 12 regional vaccination centers were involved for a period of 12 months (data referring to 2017) in which a total of 3.936 first doses of MPRV vaccine were administered (from the 13th at the 23rd month of life). Of these, 1.672 children were recruited, i.e. 42,5% of the subjects who received the first dose of the MPRV vaccine, to participate in the regional vaccination programme. Active pharmacovigilance.
The Apulian study is of particular importance since it is not limited to analyzing only the data of 1.672 subjects, but correlates the trend of reports between active and passive Pharmacovigilance and what emerges is of enormous interest.
Let's now just talk about the study on the Priorix Tetra vaccine
By analyzing the data from the MPRV vaccine alone and comparing the passive Pharmacovigilance of the period from 1 January 2013 to 31 December 2017, with the active Pharmacovigilance of the 12 months of the Study, the question becomes extremely interesting: in 5 years, 296.617 doses of MMRV vaccine were administered and 112 were observed adverse reactions by passive Pharmacovigilance (0,02%).
In the 12 months of the active Pharmacovigilance study, out of 1.672 children who were administered the first dose of MPRV, 656 adverse reactions (39.23%) were observed.
Let's look at it in the table to understand better:
| Doses administered | Adverse events detected | Reporting rates |
| 296.617 | 112 (0,02%) | 0,42x1.000 doses |
| 1.672 | 656 (39.23%) | 392,34x1.000 doses |
This data alone should make us understand how passive Pharmacovigilance is seriously deficient given the enormous difference: the adverse reactions collected with active Pharmacovigilance exceed the reports collected by 339 times spontaneously with passive Pharmacovigilance!
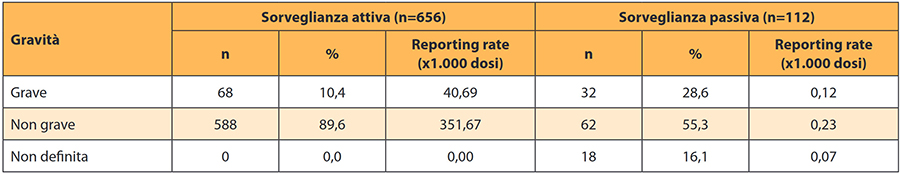
The data that emerged is even more worrying if looked at in detail. By evaluating the ability to detect adverse events with the two Pharmacovigilance systems based on their severity, we realize that the reports of Serious adverse events related to vaccination had a reporting rate of 40,69x1.000 doses in active Pharmacovigilance and only 0,12x1.000 doses with passive. This means that 4 out of 100 children, recruited with active Pharmacovigilance, had a serious adverse reaction while only 1 in 12.000 children was detected with passive Pharmacovigilance. Does this seem statistically acceptable to you?
Note An adverse event from a vaccine or drug is classified as serious if it has resulted in: death, life-threatening, serious or permanent disability, congenital anomalies/deficit of the newborn, hospitalization or prolongation of hospitalization, other clinically relevant condition.
Below is the table on page 26 which summarizes this data:
Who reports?
The report from the Puglia Region provides us with another couple of very interesting data: the subjects recruited for the study Active pharmacovigilance had reports only from pharmacists (99,6% of the total reports) while those from Passive pharmacovigilance they are divided into 10,6% doctor, 8% patient or other professional figure, 23,4% pharmacist and 58% other healthcare worker. In fact, the Apulian report highlights that doctors tend not to report suspected adverse reactions and this is of an unprecedented severity - also considering the fact that they are legally obliged to do so - and we can tell you that the situation is similar in almost all of Italy.
The ability to detect adverse reactions, in a healthy system, should belong above all to doctors, professional figures capable of observing, managing and possibly remedying an adverse reaction, but evidently ours is not a healthy system.
Not only that, the study from the Puglia Region shows us how vaccines are almost always administered in co-administration with other vaccines. Considering only the MPRV vaccine, there is a constant co-administration with the anti-meningococcal B or with the DTP (Diphtheria-Tetanus-Pertussis) or anti-polio and this makes it even more difficult to understand, identify and report the potential cause of the suspected adverse reaction.
Passive pharmacovigilance in the Apulian report
However, the complete report from the Puglia Region takes into consideration all reports of adverse events after vaccination (any vaccination in any age group) from 1 January 2013 to 31 December 2017 included in the National Pharmacovigilance Network, therefore deriving from both active and passive pharmacovigilance.
Here too we notice something very interesting: the total number of reports was 871 out of a total of 6.965.852 vaccine doses administered at a regional level, with a reporting rate equal to 12,5x100.000 doses (of which 19,4% classified as serious).
If we took these numbers at face value - 871 adverse reactions out of almost 7 million doses administered in 5 years - the safety of vaccination practice would seem undoubted, at least in the Puglia region... but as we have just seen, these are not realistic numbers!
Unfortunately, we are seeing where the data on vaccine safety in Italy are extrapolated from: this general report, despite containing alarming evidence within it, namely the total unreliability of passive pharmacovigilance in determining the safety of vaccines, offers as a conclusion - to a superficial reading - just the safety profile of these products!
Let's play with the numbers and do a prospective analysis of hypothetical active pharmacovigilance
If they had only activated Pharmacovigilance active from January 1, 2013 to December 31, 2017, how many adverse reactions could they have identified? Let's try to think about it.
Using data from the Puglia Region study, we know that the total doses of the MPRV vaccine administered were 296.617. We also know that the study compared only the first doses and considering the National Vaccine Prevention Plan 2017-2019,(4) we can assume that all the subjects had the two doses required by the vaccination calendar. It can be deduced that approximately 148.000 first doses of the MPRV vaccine were administered and we will make our calculation on these.
With 148.000 first doses of Priorix Tetra vaccine (MPRV) inoculated in 5 years, using the same reporting rate as the 1.672 children collected with active Pharmacovigilance in 2017, they should have detected from 37.000 to 58.000 suspected adverse reactions, of which a few thousand were serious.
We know very well that this calculation has no statistical value, but it still seems better than the one used to decide health policies in Italy.
References (click to open)
- https://www.sanita.puglia.it/documents/36126/4921952/Sorveglianza+degli+eventi+avversi+a+vaccino+in+Puglia+Report+2013-2017/
- https://www.corvelva.it/approfondimenti/bugiardini/mpr-mprv-morbillo-rosolia-parotite-varicella.html
- https://www.corvelva.it/malattie-vaccini/facciamo-il-punto/la-farmacovigilanza-un-fallimento-tutto-italiano.html
- https://www.salute.gov.it/imgs/C_17_pubblicazioni_2571_allegato.pdf