Summary - Data confirmation by interlab analysis

Dear friends,
we are introducing this report with a different and more emotional state of mind, as it is the last one for 2019, and we have come to the end of what we had planned for this year.
The report is a document Which summarizes the works we have done, and it just Confirms what before was hypothetical, and has now become reliable date.
We have finished our funds for this project, and we feel extremely satisfied, because empty pockets in these hard times means that all our energies have been used for the cause, without any spares.
As happened since 2017, we will auto-finance our projects thanks to the membership fees and donations, and we will go on in 2020, With a whole series of investigations That Should take the issue to a higher level; in the meantime, we will dedicate the next few months to inform and interact with foreign associations to proceed with the international complaint. We have a lot of work, honestly there are more ideas than the energies and economic possibilities available.
Together we have started a journey that has lasted a long time, and today we have succeeded in making the results of this project known to the whole world. Our determination, as parents and Italian citizens, is today an example for many other nations That unfortunately are getting ready to fight the same battles in defense of fundamental human rights.
Yes, it is very true, the institutions appear deaf and no comma has moved yet, at least not in a clear manner; most distressingly, our children are excluded from schools, but let's look around: the world system gravitates around the vaccination dogma supported by a "scientific community" that, in Italy alone, has received more than half a billion euros in a few years. 1 It is a veritable lot of money that has moved a host of pseudo-scientists, hired doctors and slothful politicians, ready to protect the interests of those entities that Prof. Randy Schekman, Nobel Prize for medicine in 2013, called "caste". 2
"Science is at risk: it is no longer reliable because it is in the hands of a closed caste and anything but independent" and this is now evident even to the most skeptical of spectators - if in good faith. It pursues interests that are not those that accompany the scientific method, at least not as it should be; produces studies that, in order to be published, must comply with some imposed dogmas, omitting some data that "do not agree" or highlighting others that instead "are convenient" and this is something we have experienced directly, although we are only overlooking this world.
In this despicable situation, we found ourselves having to face a legal imposition for the administration of pharmaceutical products, a law that enriches and increases the power of that caste of which Nobel Schekman speaks.
In front of such a socio-political situation we, as aware and determined citizens and parents, have strengthened ourselves, with the help of other citizens like us; we have taken those actions that seemed more effective or simply more "affordable", based on our possibilities: we have shown that with little strength, but with determination, we can produce great results.
We started with these few opening lines because today, as the latest Corvelva report, we will talk about the latest results, which are confirmations arrived through analyzes carried out both by a prestigious European university and by other certified laboratories located in different parts of the world . The date is always more certain and probable what was before is now Demonstrated!
Among other things, we have investigated a few toxic and/or carcinogenic compounds, such as NDMA nitrosodimethylamines and cyanohydrins, and the results have been really troubling. We will necessarily talk about this with peer review publications in hand, in order not to compromise the publications themselves.
As explained, these in-depth studies, as well as those about fetal DNA sequencing, are the subject of the next peer review publication, so we are really communicating a small part of what will be published, but this is what science asks, that the data is new. If we'd report more details, we would put the work done. The fact remains that the control bodies received everything, in the original!
We will proceed with information and we will fight so that the disturbing results are considered for what they are, pure analytical data without preconceptions or ideologies. The products analyzed by us are to be withdrawn in mass and there is a great question to be asked about the interests that gravitate around all those who have placed themselves against our analyzes and / or in favor of vaccination dogma.
Together we can try to stop all this madness.
Summary of results confirmed Obtained for each vaccine
In order to carry out this survey, it was DECIDED to use a cut-off between nanograms and micrograms, Therefore above the residual limit for unknown compounds not reported in the technical data sheet.
It should be emphasized that the cumulative quantity of these contaminants is above micrograms per dose, although it is not possible to make an exact quantification at the moment, as most of the contaminants are known and therefore it is not possible to carry out the study using analytical standards control.
From the results of the screening and the controls with the standards, it appears that the metabolic fraction shows candidate compounds whose presence can only be explained with a poor control, and, consequently, with the poor quality of the raw materials and reagents. In particular, cross-contamination with chemical compounds with known pharmacological activity is out of control, although it is possible that the vaccine production process will change its structure and original conformation.
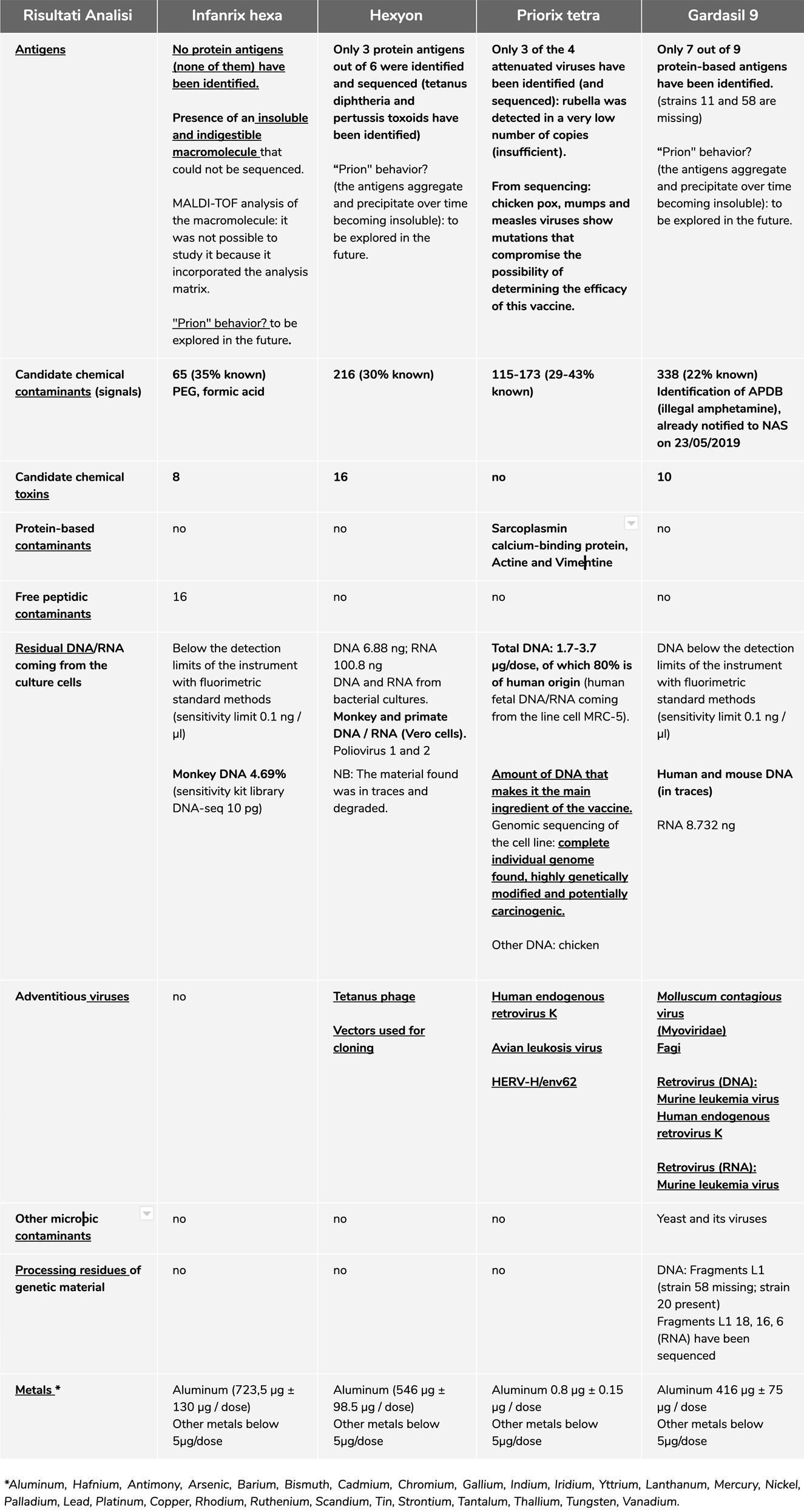
Specification of confirmed results
At the bottom of this summary, we will report all the links of all the reports of the analyzes performed, please always refer to them for a detailed understanding of the results, since here below we will talk only about the confirmatory analyzes through second level screening. , control standards and / or interlaboratory.
An example to better understand: a certified service provider has performed some analysis upon our request. They were asked to work on targets, to identify the presence or absence of some organic or inorganic compounds in certain vaccines (not all of them) or genetic sequences. Therefore, the result is only about what we have asked to look for, which is an indispensable element to confirm the validity of the method used by us.
The reason for not having used numerous control standards and other vaccines on the market has economic and logical roots: we are not a research institution and we are doing in-depth studies. After having reported the non-conformity to the regulatory bodies, a fact that goes beyond what we should compete, to all intents and purposes we are inappropriately replacing the regulatory bodies themselves in investigations that would be up to them after a report of this magnitude, and we're doing it because our data has been called incomplete.
Hexyon® vaccine analysis
metagenomic
The presence of the contaminating genetic material was confirmed with the interlaboratory analysis at a European certified service provider.
The DNA present is equal to 6.88 ng total per dose (this quantity refers to the disclosed report. The data of the interlaboratory analysis is subject to peer review and therefore not disclosed, but confirms the order of magnitude), of which 0, 1% potentially coming from Vero cells (Cercopithecidae), that is 688 pg / dose. We Identified Clostridium phage phiCT453A and SV40 together with other vectors for cloning.
Presence of Polioviruses 1 and 2. In this case, the EMA's response to the absence of Poliovirus 3 was very generic: the absence of Poliovirus 3 is not a non-conformity for them, because they consider the presence of the antigen D, which is able to create immunization. Obviously, we have searched for this protein, but we have not been able to find it, it would be an excellent in-depth analysis to develop because we currently leave an uncertain answer on this point.
DNA and RNA of bacteria used to produce Corynebacterium diphtheriae (Diphtheria), Clostridium tetani (Tetanus), Bordetella pertussis (pertussis) and Haemophilus influenzae antigens.
NOTE: the adventitious genetic material present in the vaccine can be linked to the adjuvant aluminum with possible enhancement of toxic effects (inflammatory, autoimmune and tumor capacity). We reiterate That, from the Interlaboratory confirmed date, the safety and efficacy of this vaccine remains doubtful, Resulting in a completely non-compliant product with reference to quality.
Gardasil 9® vaccine analysis
Chemical protein
After the previous results, we DECIDED to investigate further, with inter-laboratory confirmation, the identification of the compound APDB (illegal amphetamine), Already Notified to the NAS on May 23, 2019. Two different laboratories confirm the presence of a substance Belonging to the class of illegal APDBs.
We repeat it again: we cannot buy the standard of control, being the APDB classified as narcotic, 3 it cannot be acquired by persons without specific authorization, therefore we have provided all the documentation that confirms the presence of a substance belonging to the APDB class and the possible origin of the contamination (Note: for the registration of Gardasil 9® the EMA assessment reports that L-tyrosine is used as a raw material for the production of this vaccine and is extracted from human hair from China. 4 The main production of this narcotic addicts comes from China and have a very high level in their hair. 5
metagenomic
The presence of genetic material has been confirmed by inter-laboratory analysis at a European certified service provider and we can repeat the previous data, they found:
- Human and mouse DNA (below the detection limits of the instrument);
- Adventitious viruses;
- L1 fragment of double-stranded DNA HPV virus;
- Phages;
- Molluscum contagiosum virus;
- Retroviruses:
-
- Murine leukimia virus;
- Human endogenous retrovirus K;
- saccharomyces
NOTE: the genetic adventitious material found in the vaccine can be bound to the adjuvant aluminum with possible enhancement of the toxic effects (inflammatory, autoimmune and tumorous capacity)
Priorix Tetra® vaccine analysis
metagenomic
The presence of genetic material has been confirmed by inter-laboratory analysis at a European certified service provider.
The quantities refer to the reports disclosed. The data of the inter-laboratory analysis are subject to peer review and therefore not disclosed but they confirm the order of magnitude.
DNA - The total amount of DNA present in this vaccine ranges from: 1.7 - 3.7? G / dose and it is in effect the main component of the vaccine. There is about 80% of human DNA (74-88%) and chicken DNA (0-4%).
The human genome is complete, that is with non-coding genes and sequences, of high molecular weight, male, qualified as belonging to the fetal line MRC-5 that is the continued cell line derived from pulmonary tissue of male abortive fetus of the 60s . The sequencing of this cell line has proven to be highly genetically modified and potentially carcinogenic. The sequencing analysis of the whole fetal DNA genome was performed at an American service provider (laboratory).
RNA - Human 68-87%. Chicken 0-0.2%
Attenuated viruses - The following attenuated viruses have been confirmed lot. A71CB256A:
- Chickenpox (DNA) 11%;
- Mumps (RNA) 0.008%;
- Measles (RNA) 0.004%;
- Rubel 0.00004%. (114 out of 260 million sequences)
An irrelevant presence of rubella in the vaccine (lower than the adventitious viruses shown below) was confirmed inter-laboratory. This seriously undermines the effectiveness of the vaccine.
Viral quasi-species: 245 variants have been identified in the vaccine varicella genome with respect to the reference genome used for the analysis (wild genome of the Dumas strain). Of these variants, 154 are major variants while the remaining 91 are quasi-species variants. There is no difference between the variants found in the two lots. In the mumps vaccine genome, 40 quasi-species variants have been identified with respect to the reference genome used for the analysis (Jeryl-Lynn vaccine genome), the comparison between the variants found in the two lots highlights 4 differences. The EMA was not able to provide us with the vaccine virus sequences used by the manufacturer for this vaccine, as they are covered by trade secrets, which is why we do not know how much vaccine viruses have changed compared to what the manufacturer declared.
Due to low coverage it was not possible to detect quasi-species variants for measles and rubella genomes.
Adventitious viruses - We have confirmed the presence of these adventitious viruses:
- Human endogenous retrovirus K;
- Avian leukosis virus;
- HERV-H / env62.
For all vaccines
Analysis on chemical contaminations with control and inter-laboratory standards
Two compounds were chosen to be analyzed with certified control standards, based on availability, the consistency of the semi-quantitative data and the impact on health. These compounds have been confirmed as similar (ie. With a 75 - 80% structural identity: isomers / isobars) also by inter-laboratory analysis. The structure of these compounds will be disclosed with the peer review publication.
Second level screening on chemical contaminations
The second level screening survey, as already mentioned, is divided into three analyses:
- in-depth screening of the submerged part (65% of the total signals) and comparison with the database of the detected substances, including the toxins database.
- Analyzes of neutral losses: the detection of stable neutral fragmentations allow us to hypothesize the presence in the vaccine of molecules that contain them, of unknown structure but with potential toxic effects if the functional groups have a carcinogenic and mutagenic activity [the “Cohort of concern” created by the EMA for oral drugs includes aflatoxin-like compounds, N-nitroso- and alkyl-azoxy, as reported in the ICH guideline M7 (R1). 6
New candidate compounds: potentially toxic compounds were detected for all vaccines Examined (cross-contamination) based on comparison with databases. It would be useful to investigate it by using checking standards to confirm its identity.
Neutral losses: in our case the cyanyl groups (ie. Derived from the cyanide acid used to prepare the Haemophilus B vaccine) and the nitrosodimethylamine, a carcinogenic impurity found in other drugs and subject to evaluation by the EMA, were examined. 7
In order to understand the functional group of nitrosodimethylamines, consider that it is of the same class as the contaminations found in the Sartan and ranitidine drugs leaping to the news for the recent massive withdrawal of drugs throughout Europe. 8 The second functional group is that of cyanides, of which we know the declared presence of sodium cyanide as shown in the technical data sheets of the Prevenar vaccine. 9
Second-level screening therefore gave a positive response for all vaccines. It is not possible at the moment to disclose the results in detail because they're in the peer-review publication phase, but all regulatory bodies have been made aware of our results.
Thelegislation
Please note what must be done by law on commercial drugs: 10
The Annual Control Program makes it possible to guarantee that the drugs sold correspond exactly to the quality specifications of the authorization procedures. It is established every year by the AIFA, after having heard the opinion of the Istituto Superiore della Sanità, and is approved by the Technical Scientific Committee (CTS) of the AIFA. The AIFA Product Quality Office requires the NAS (Unit (of the Carabinieri) responsible for preventing the adulteration of foodstuffs, drugs and beverages) to take samples of the medicines provided in the program, which are sent to the ISS for analysis at pharmacies or wholesalers . If the results of the analysis reveal differences from what has been authorized, the AIFA Product Quality Office takes the necessary measures. The analyzes carried out are based on the verification of compliance with the quality specifications authorized for each drug and reported in the registration dossier and / or in the European Pharmacopoeia monographs. (...)
The Product Quality Office also manages the office revocation and suspension of marketing authorizations.
The AIC of a medicinal product can be revoked, with consequent definitive withdrawal from the market when:
- the medicine is harmful under normal conditions of use;
- the medicine does not have the therapeutic effect or the effect for which it was authorised;
- the risk / benefit ratio is not favorable under normal conditions of use;
- the medicine does not have the declared qualitative and quantitative composition;
- the medicine was produced in unauthorized establishments.
The authorization can be revoked even in case that the information in the application for authorization of the medicine is found to be not correct or in the absence of controls on the finished product, components or intermediate products of production.
In addition to the interventions adopted at national level, the measures of the Rapid Alert System, defined on the basis of shared European procedures, are carried out at the same time; these consist of different actions and notifications according to the type of seriousness of the defect in accordance with the classification of urgencies.
Figure 2 describes the classification of defects and the related actions to be taken
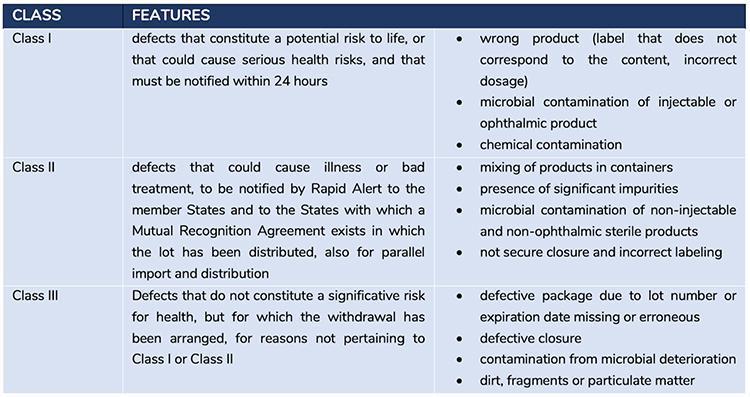
Please note that the defects detected with the analysis carried out by Corvelva fall into Class I and II, as the vaccines are drugs for injective use and the contaminations detected are of a chemical and genetic material type.
We underline that, up to date, Has Been no measure taken by the regulatory agencies, despite the notification to AIFA of the results of the preliminary analyzes and to the NAS of the presence of the APDB compound in the Gardasil 9® vaccine: this is in serious violation of the precautionary principle and of the need for rapid intervention, as foreseen by the Alert System for the protection of public health.
Download: CORVELVA-Summary-Data-confirmation-Interlab-analysis.pdf
Note:
- https://www.corvelva.it/it/approfondimenti/sistema-sanita/case-farmaceutiche/efpia-italia-tutti-i-trasferimenti-di-valore-delle-big-pharma.html
- http://www.medicinapiccoledosi.it/medicina-convenzionale/premio-nobel-la-medicina-randy-schekman-la-scienza-mano-ad-casta/
- http://www.cortedicassazione.it/cassazione-resources/resources/cms/documents/Legge_79_2014.pdf
http://www.rivistagiuridica.aci.it/fileadmin/Documenti/Decreto_25_giugno_M_Salute_02.pdf - https://www.unodc.org/documents/scientific/Trends_and_Patterns_of_ATS_and_NPS_2017.pdf
- Gas Chromatography-Mass Spectrometry (GC-MS) AnalysisJournal of Food and Drug Analysis, Vol. 13, No. 3, 2005, Pages 193-200 Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Amphetamine, Methamphetamine, 3,4-Methylenedioxy- amphetamine and 3,4-Methylenedioxymethamphetamine in Human Hair and Hair SectionsDONG-LIANG LIN1,2 *, REA-MING YIN1 AND RAY H. LIU3
- https://www.ema.europa.eu/en/documents/ … /ich-guideline-m7r1-assessment-control-dna-reactive-mutagenic-impurities-pharmaceuticals-limit_en.pdf
- http://www.agenziafarmaco.gov.it/content/comunicazione-ema-sul-principio-attivo-valsartan-19112018
- https://www.repubblica.it/salute/medicina-e-ricerca/ … /news/non_solo_ranitidina_per_l_ema_vanno_testati_tutti_i_farmaci_per_impurita_cancerogene-237010173/
- https://www.ema.europa.eu/en/documents/scientific-discussion/prevenar-epar-scientific-discussion_en.pdf
- http://www.bollettinosifo.it/r.php?v=2598&a=26744&l=329640&f=allegati/02598_2016_06/fulltext/05_EspOpin_Cannizzo.pdf

